The molar heat capacity for an ideal gas
1. cannot be negative
2. must be equal to either or
3. must lie in the range
4. may have any value between and
An ideal gas expands according to the law = const. The molar heat capacity C is :
1.
2.
3.
4.
The molar heat capacity C for an ideal gas going through a given process is given by C = a/T , where 'a' is a constant. If , the work done by one mole of gas during heating from to through the given process will be:
1.
2.
3.
4. none of these

The pressure of a monoatomic gas increases linearly from N/m2 to N/m2 when its volume increases from 0.2 m3 to 0.5 m3. The molar heat capacity of the gas is:
[R = 8.31 J/mol k]
1. 20.1 J/molK
2. 17.14 J/molK
3. 18.14 J/molK
4. 20.14 J/molK
An ideal gas undergoes a cyclic process as shown in the graph between pressure \((P)\) and temperature \((T).\) The part of the graph which represents the absorption of heat from the surrounding are:
1. \(\text{AB, CD}\)
2. \(\text{DA, BC}\)
3. \(\text{DA, AB}\)
4. \(\text{BC, AB} \)
Ideal gas is taken through cycle as shown in the figure. If net heat supplied to the gas in the cycle is 15 J, the work done by the gas in the process is
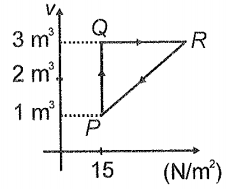
1. 5J
2. -15 J
3. -20 J
4. -12 J
Internal energy remains the same throughout the process along the path in
1 Adiabatic process
2 Isothermal process
3 Cyclic process
4 Both (2) & (3)
The cyclic process of \(2\) moles of diatomic gas is shown in the figure. Which of the following statements is correct?

| 1. | The process \(BC\) is an isothermal compression. |
| 2. | The work done in the process \(CA\) is \(4 R T_0\) |
| 3. | The work done in the process \(AB\) is zero. |
| 4. | All of these |
Without external heat work done possible in
1 Adiabatic process
2 Isochoric process
3 Isothermal process
4 Isobaric process







