What is the best description of the geometry of the nitrogen atoms in dimethylnitrosamine, ?
N bounded to groups N bonded to O
1. Trigonal planar Linear
2. Trigonal planar Bent
3. Trigonal pyramidal Linear
4. Trigonal pyramidal Bent
The following diagram is sometimes used to illustrates the structure of benzene
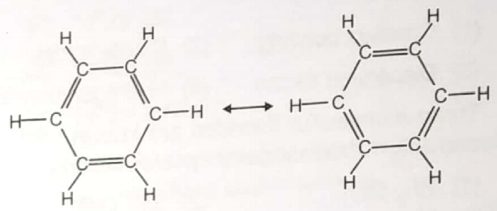
Which of the statements concerning the structure of benzene is false?
1. The double bonds oscillate rapidly back and forth between adjacent pairs of carbon atoms
2. The
3. The carbon atoms form a flat hexagonal ring
4. Above structures are resonance structures
Consider the Lewis structure given below for the molecule
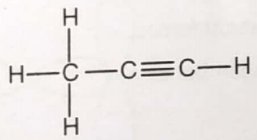
What is the maximum number of atoms that can lie in the same plane?
1. Three
2. Four
3. Five
4. Six
How many carbon-carbon double bonds are present in linolenic acid, ?
1. 1
2. 2
3. 3
4. 4
The formulae and boiling points of three compounds are given in this table.
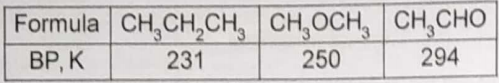
The trend in boiling points is best attributed to variations in
1. Covalent bonding
2. Dipole forces
3. Dispersion forces
4. Hydrogen bonding
Three monosulfur fluorides are known : . Of these, polar species include
1. Only
2. Only
3. and Only
4. , and
Which ionic compound has the largest lattice energy?
1. LiF
2. BeO
3. KBr
4. CaS
The boiling points of are , respectively. This increase is best attributed to an increase in which of the following?
l. Dipole-dipole interactions.
ll. Dispersion forces.
lll. Hydrogen bonding.
1. l only
2. l and ll.
3. lll only
4. ll and lll only
The lattice energy (energy required to separate the ions in an ionic solid) of MgO is much larger than that of LiF.
What contributes the most to this difference?
1. is a smaller ion than and is a smaller ion than
2. F is more electronegative than O, and Li is more electropositive than Mg
3. MgO contains doubly charged ions, while LiF contains singly charged ions
4. MgO contains more electrons than LiF






