The bond order for a species with the configuration will be
1. 1
2.
3. Zero
4. 3/2
Subtopic: M.O.T |
84%
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
Which molecule is least likely to form hydrogen bonds?
| 1. | \(\mathrm{NH}_3\) | 2. | \(\mathrm{H_2NOH}\) |
| 3. | \(\mathrm{HF}\) | 4. | \(\mathrm{CH_3F}\) |
Subtopic: van der Waal Force & Hydrogen Bonding |
63%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
The correct sequence of stability for the given molecular species (from the given options) is:
1.
2.
3.
4.
Subtopic: M.O.T |
71%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
From the given structures, the correct structure(s) of is are
l. 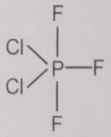
ll. 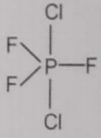
lll. 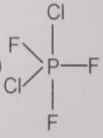
1. Only l
2. Only ll
3. Only lll
4. l, ll and lll
Subtopic: Hybridisation |
63%
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
The pair that is isostructural (i.e. having the same shape and hybridization) is
1.
2.
3.
4.
Subtopic: Hybridisation |
88%
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints






