Which of the following represents the correct order of dipole moment?
1.
2.
3.
4.
Lattice energy for an ionic compound is calculated by using
1. Kirchhoff's equation
2. Markownikoff's rule
3. Born Haber cycle
4. Carnot cycle
Assuming that Hund's rule is violated by the diatomic molecule , its bond order and magnetic nature will be respectively
1. 1, diamagnetic
2. 1, paramagnetic
3. 2, diamagnetic
4. 2, paramagnetic
In the Lewis structure of ozone the formal charge on the central oxygen atom is
1. +1
2. -1
3. 0
4. -2
For , the molecular geometry and hybridization of the central atom respectively are
1. Square planar,
2. Tetrahedral,
3. Seesaw,
4. Square pyramid,
A diatomic molecule has a dipole moment of 1.2 D. If its bond length is equal to 10 -10 m then the fraction of an electronic charge on each atom will be:
1. 42%
2. 52%
3. 37%
4. 25%
There is no S - S bond in :
(A) (B)
(C) (D)
The pair that is isostructural (i.e. having the same shape and hybridization) is
1.
2.
3.
4.
From the given structures, the correct structure(s) of is are
l. 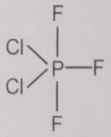
ll. 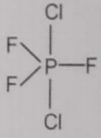
lll. 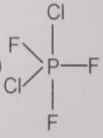
1. Only l
2. Only ll
3. Only lll
4. l, ll and lll






