Consider the following reaction sequence
 HNO3+H2SO4→ Sn+HCl→NaNO2+HCl→(6H)Cu2(CN)2+HCN→H2O→Product
HNO3+H2SO4→ Sn+HCl→NaNO2+HCl→(6H)Cu2(CN)2+HCN→H2O→Product
Product is
(1)

(2)

(3)

(4)

 HNO3+H2SO4→ Sn+HCl→NaNO2+HCl→(6H)Cu2(CN)2+HCN→H2O→Product
HNO3+H2SO4→ Sn+HCl→NaNO2+HCl→(6H)Cu2(CN)2+HCN→H2O→Product



CH3Cl →CH4
Above conversion can be achieved by:
(a) Zn/H+
(b) LiAlH4
(c) Mg /(ether) then H2O
(d) all of these
CH3-CH2-CH2-CH3 AlCl3/∆→ CH3-CH-CH3
l
CH3
Above reaction is an example of:-
(a)isomerization (b)polymerization (c)cracking (d)de-hydrogenation

Product (A) of the reaction is :
1. CH3-CH3
2. CH2=CH2
3. CH3-CH=CH2
4. none of these
Which of the following bromides is the major product of the reaction shown below, assuming that there are no carbocation rearrangement?
(1 equivalent)
1.
3.
Compound (A) is :
1. 2.
3. 

1. 
2. 
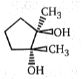
3. 
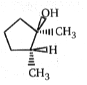
4. 
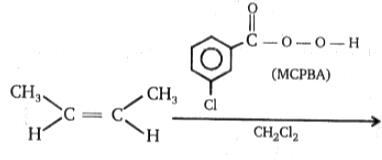 Product; Product is:
Product; Product is:
MCPBA →Metachloroperbenzoic acid
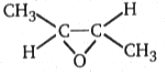
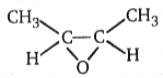
1. 
2. 
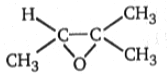
3. 
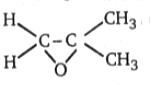
4. 
What is the major product expected from the following reaction?
D-Cl→ Product
1.
2.
3. 
4.
CH3-C|H-CO2K
CH3-CH-CO2K electrolysis→ (A) (Major)
Major product (A) of the above reaction:















