. The concentration of free ions in a solution of the complex ion can be increased significantly by the addition of
1.
2. HCl(aq)
3.
4.
Given the following pair of isomers:
[Cr(NH3)6][Cr(NO2)6] and
[Cr(NH3)4(NO2)2][Cr(NH3)2(NO2)4]
Aside from X-ray diffraction, how could the following pairs of isomers be distinguished from one another?
1. Cryoscopic method
2. Measuring their molar conductance
3. Measuring their magnetic moments
4. Observing their colors
The hybridizations of , respectively, are :
1.
2.
3.
4.
Which of the following is true about the complex ; [Atomic number of Pt = 78]
I. It will have two geometrical isomeric forms, cis and trans
II. The hybridization state of Pt(ll) is
III. It is a square planar complex
IV. It is a diamagnetic complex
V. It can show hydrate isomerism
VI. It is a tetrahedral complex
1. I, III, IV
2. II, IV, V
3. II, V, IV
4. I, V, IV
Correct statement among the following regarding , and is -
1. and are diamagnetic and is paramagnetic
2. and are diamagnetic and is paramagnetic
3. and are diamagnetic and is paramagnetic
4. is diamagnetic and and are paramagnetic
Among the following statements, the correct statement is/are -
a. and have the same value of CFSE
b. and have the same value of the magnetic moment
c. and have the same hybridization of nickel
1. a & c only
2. b & c only
3. a & b only
4. All of the above
Which is the correct electronic configuration for the complex ?
1.
2.
3.
4.
A compound is made by mixing cobalt (III) nitrate and potassium nitrite solution in the ratio of 1 : 3. The aqueous solution of the compound showed 4 particles per molecule whereas molar conductivity reveals the presence of six electrical. charges. The formula of the compound is
1.
2.
3.
4.
0.001 mole of was passed through a cation exchanger and the acid coming out of it required 20 ml of 0.1 M NaOH for neutralization. Hence, the complex is
1.
2.
3.
4. All of these
Which of the following statements is correct regarding the chirality (optical isomerism) of the cis and a trans isomer of the type (M stands for metal, aa is a bidentate ligand, and b is a chiral ligand)?
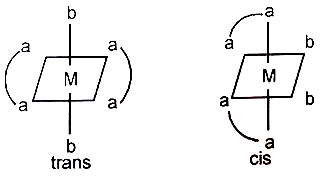
(1) The trans form is achiral and optically inactive while the cis form is chiral and exists in two enantiomeric forms
(2) Both cis and transforms are achiral and optically inactive
(3) The transform is chiral and exists in two enantiomeric forms while the cis form is achiral and optically inactive
(4) Both the cis and transform are chiral and each exists in two enantiomeric form






