The crystal field stabilization energies (CFSE) of high spin and low spin metal complexes in terms of , respectively are
1. -0.4 and -2.4
2. -2.4 and -0.4
3. -0.4 and 0.0
4. -2.4 and 0.0
The oxidation state of cobalt in the following molecule is
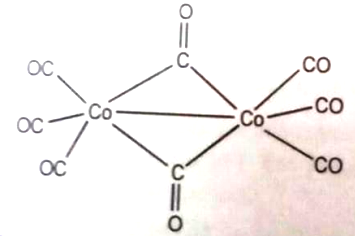
1. 3
2. 1
3. 2
4. 0
For a tetrahedral complex [MCl4]2-, the spin-only magnetic moment is 3.83 B.M. The element M is:
1. Co
2. Cu
3. Mn
4. Fe
Among the following, the species that is both tetrahedral and diamagnetic is
1.
2.
3.
4.
The number of ions produced in water by the dissolution of the complex having the empirical formula is
1. 1
2. 2
3. 4
4. 3
In aqueous solution, (X) reacts with molecular oxygen in the presence of excess liquor to give a new complex Y. The number of unpaired electrons in X and Y are, respectively
1. 3,1
2. 3,0
3. 3,3
4. 7,0
The number of geometrical isomers of , where en = ethylenediamine, is
1. 2
2. 3
3. 4
4. 1
The energies of orbitals in octahedral and tetrahedral transition metal complexes are such that
1. in both tetrahedral and octahedral complexes
2. in both tetrahedral and octahedral complexes
3. in tetrahedral but in octahedral complexes
4. in tetrahedral but in octahedral complexes
The geometry and the number of the unpaired electron(s) of , respectively, are
1. Tetrahedral and 1
2. Square planar and 1
3. Tetrahedral and 5
4. Square planar and 5
Among the following complexes, the one that can exhibit optical activity is
1.
2.
3.
4. trans-






