Sodium ethoxide reacts with ethyl iodide to yield:
1. CH3CH3 2. C2H5OCH3
3. C2H5OC2H5 4. none of these
The set of reagents used to produce freon (CCl2F2) are:
| 1. | C + F2 + Cl2 → |
| 2. | CH3Cl + F2 → |
| 3. | \(CCl_4 + HF \ \xrightarrow[]{SbCl_5} \) |
| 4. | CCl4 + F2 → |
What causes an alkyl iodide to darken on standing?
1. Hydrolysis
2. Conversion into ether
3. Liberation of iodine
4. Formation of alkanes
In the following sequences of reactions;
CH3CH2CH2I →KOH(alc.)(A)→Br2(B)→NaNH2/NH3(C) the end product (C) is:
1. alkene
2. alkanol
3. alkyne
4. alkyl amine
PCI5 reacts with propanone to give:
1. gem-Dichloride
2. vic-Dichloride
3. Propanal
4. Propane chloride
Identify (Z) in the following reaction series,
C2H5I (x) (Y) (Z):
1. CH3-CH2-CN
2.
3.
4.
Identity ‘Z’ in the following reaction series,
aq. NaOH Al2O3(Heat) HOCl
CH3.CH2CH2Br→ (X) → (Y) →(z)
1. Mixture of
CH3-CH-CH2 and CH3-CH-CH2
l l l l
Cl Cl OH Cl
2. CH3-CH-CH2
l l
OH Cl
3. CH3-CH-CH2
l l
Cl OH
4. CH3-CH-CH2
l l
Cl Cl
A compound A of formula C3H6Cl2 on reaction with alkali can give B of formula C3H6O or C of formula C3H4. B on oxidation gave a compound of the formula C3H6O2. C with dilute H2SO4 containing Hg2+ ion gave D of formula C3H6O, which with bromine and NaOH gave the sodium salt of C2H4O2. Then A is:
1. CH3CH2CHCl2
2. CH3CCl2CH3
3. CH3CHClCH2Cl
4. CH2ClCH2CH2Cl
Which chloro derivative of benzene among the following would undergo hydrolysis most readily with aqueous NaOH to furnish the corresponding hydroxy derivative?
1.
2.
3.
4.
Which of the following compounds are optically active?
(i). 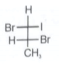
(ii). 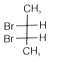
(iii). 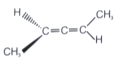
(iv). 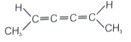
1. (i) & (ii)
2. (i) & (iii)
3. (ii) & (iii)
4. (i) & (iv)









