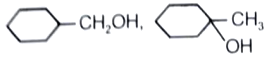On boiling with concentrated hydrobromic acid phenyl ethyl ether yields:-
1. Phenol and ethyl bromide
2. Bromobenzene and ethanol
3. Phenol and ethane
4. Bromobenzene and ethane
An organic compound A reacts with sodium metal and forms B. On heating with conc. H2SO4, A gives diethyl ether. So A and B are [AFMC 1998]
1. C3H7OH and CH3ONa
2. CH3OH and CH3ONa
3. C4H9OH and C4H9ONa
4. C2H5OH and C2H5ONa
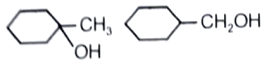
Identify A and predict the type of reactions
1. 
2.
3.
4.
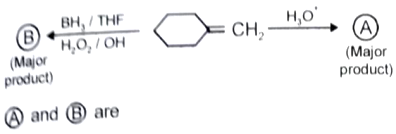
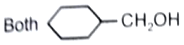
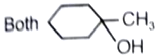
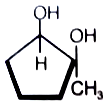
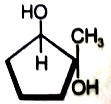
What is the major product in the given following reaction under cold condition?
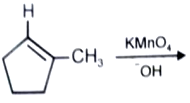
1. 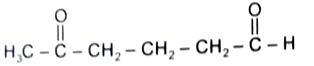
2. 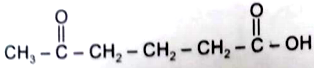
3. 
4. 
Incorrect method for the preparation of in good yield is
1.
2.
3.
4.
The electrophile involved in the above reaction is:
1. dichloromethyl cation ()
2. dichlorocarbene (:CCl2)
3. trichloromethyl anion ()
4. formyl cation ()
Phenol is weakly acidic but does not react with NaHCO3 like carboxylic acids hence:-
1. phenol is weaker than carbonic acid
2. phenol is stronger than carbonic acid
3. phenol is stronger than carboxylic acid
4. none of the above
A characteristic group test for phenolic gp. is:
1. Liebermann's nitroso reaction
2. coupling with diazonium salt
3. aqueous FeCl3 solution test
4. all of the above
Phenol on oxidation gives chloranil. The oxidant used is:
1. K2S2O8
2. KMnO4
3. KCIO3 + HCl
4. none of these