A key step in the hydrolysis of acetamide in aqueous acid proceeds by nucleophilic addition of-
1. to 
2. to 
3. to 
4. to 




The product of the below mentioned reaction is-
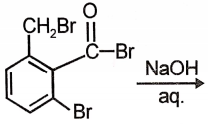
| 1. |  |
2. | 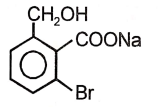 |
| 3. | 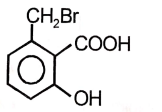 |
4. |  |
What is the correct increasing order of boiling points?
1. CH3OCH3 < CH3CHO < CH3CH2OH < CH3COOH
2. CH3CHO < CH3OCH3 < CH3CH2OH < CH3COOH
3. CH3CHO < CH3OCH3 < CH3COOH < CH3CH2OH
4. CH3OCH3 < CH3CH2OH < CH2CHO < CH3COOH
The Iodoform test is not given by:
1. CH3COCH2COOC2H5
2. CH3COCH3
3. CH3CH2COCH3
4. CH3CH2CHOHC2H5
2-Methyl propene on oxidation with hot KMnO4 gives:
1. Acetone
2. Ethanoic acid
3. CO2 and H2O
4. Both 1 & 3
Adipic acid
The compound B can be

The product 'B' in the above-mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. | CH3CH2CH2Cl |
When propanoic acid is treated with aqueous sodium bicarbonate, CO2 is liberated. The ‘C’ of CO2 comes from:
1. Carboxylic acid group
2. Methylene group
3. Bicarbonate
4. Methyl group
Which of the following will not form a yellow precipitate on heating with an alkaline solution of iodine?
1. CH3CH(OH)CH3
2. CH3CH2CH(OH)CH3
3. CH3OH
4. CH3CH2OH
Match column l with column ll:
| Column I (Name Reaction) |
Column II (Reagents) |
||
| (a) | Gattermann- Koch | (i) | CrO2Cl2 - CS2, H3O⊕ |
| (b) | Rosenmund reduction | (ii) | CO, HCl, Anhy.AICI3 |
| (c) | Stephen reaction | (iii) | H2 + Pd - BaSO4 |
| (d) | Etard reaction | (iv) | SnCl2 + HCl , H3O⊕ |
1. a(ii), b(iii), c(i), d(iv)
2. a(iii), b(ii), c(i), d(iv)
3. a(iii), b(ii), c(iv), d(i)
4. a(ii), b(iii), c(iv), d(i)







