In the compound given below,

the correct order of acidic nature of the positions (X), (Y) and (Z) is:
(a) Z>X>Y
(b) X>Y>Z
(c) X>Z>Y
(d) Y>X>Z

Aniline was acetylated and an acetanilide is formed. The acetanilide on nitration followed by alkaline hydrolysis gave:
1. o-Nitroacetanilide
2. o- and p-Nitroaniline
3. m-Nitroaniline
4. Acetanilide
In the chemical reactions,
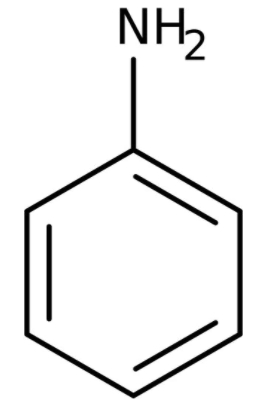
the compounds 'A' and 'B' respectively are:
1. nitrobenzene and chlorobenzene
2. nitrobenzene and flurobenzene
3. phenol and benzene
4. benzene diazonium chloride and fluorobenzene
Which of the following reactions is appropriate for converting acetamide to methanamine ?
(1) Carbylamine reaction
(2) Hoffmann Bromamide reaction
(3) Stephens reaction
(4) Gabriels phthalimide synthesis
Which one of the following nitro-compounds does not rect with nitrous acid ?
A given nitrogen-containing aromatic compound a reacts with Sn/HCl, followed by HNO2 to give an unsatable compound B.B, on treament with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N2O. The structure of compound A is
The correct statement regarding the basicity of arylamines is
(1) Arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are not delocalized by interaction with the aromatic ring p-electron system.
(2) Arylamines are generally more basic than alkylamines because of aryl group.
(3) Arylamines are generally more basic than alkylamines, because the nitrogen atom in arylamines is sp-hybridized
(4) Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring p-electron system.
Which of the following will be most stable diazonium salt ?
(a)
(b)
(c)
(d)
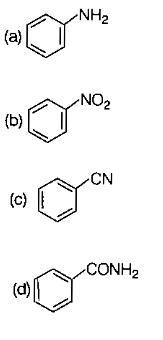
Which of the following is more basic than aniline ?
1. Diphenylamine
2. Triphenylamine
3. p-nitroaniline
4. Benzylamine
Which of the following compounds will not give Hoffmann bromamide reaction?
| 1. |  |
2. |  |
| 3. |  |
4. |  |







