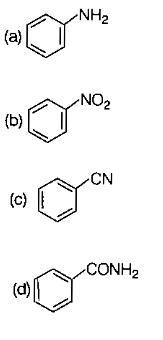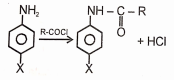
Which of the following substituent as 'X' not decreases the rate of reaction with respect to aniline?
1. 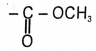
2.
3.
4. -CHO

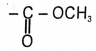
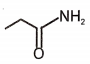
Which of the following compounds will give isocyanide test with ?
1. 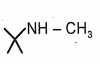
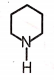
2. 
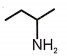
3. 
4. 
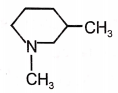
Give the incorrect order of basic strength
1.
2.
3.
4.
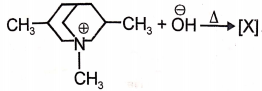
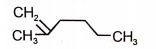
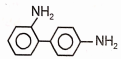
Identify the major product (A) formed in the following reaction/\.
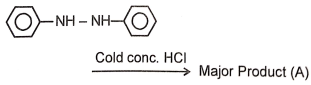
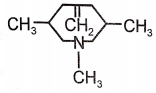
1. 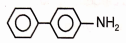 2.
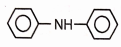
2. 
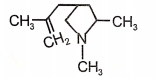
3. 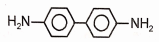 4.
4. 
Which of the following compounds will not give Hoffmann bromamide reaction?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Which of the following is more basic than aniline ?
1. Diphenylamine
2. Triphenylamine
3. p-nitroaniline
4. Benzylamine
Which of the following will be most stable diazonium salt ?
(a)
(b)
(c)
(d)
The correct statement regarding the basicity of arylamines is
(1) Arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are not delocalized by interaction with the aromatic ring p-electron system.
(2) Arylamines are generally more basic than alkylamines because of aryl group.
(3) Arylamines are generally more basic than alkylamines, because the nitrogen atom in arylamines is sp-hybridized
(4) Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring p-electron system.
A given nitrogen-containing aromatic compound a reacts with Sn/HCl, followed by HNO2 to give an unsatable compound B.B, on treament with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N2O. The structure of compound A is