How many sp and sp-hybridised carbon atoms are present respectively in the following compound ?
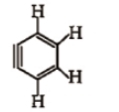
1. 4, 2
2. 6, 0
3. 3, 3
4. 5, 1

Which molecule does not exist ?

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
0.01 mole of is completely neutralized by 0.56 gram of KOH hence :
1. x = 3 and given acid is dibasic
2. x = 2 and given acid is monobasic
3. x = 3 and given acid is monobasic
4. x = 4 and given acid forms three series of salt

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following best describes the shape of \(\text{XeF}_3^+\)?
1. Trigonal planar
2. Pyramidal
3. Bent T-shape
4. See-saw

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which is the following pairs of species have identical shapes ?

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following leads to bonding orbital?
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following overlap is incorrect (assuming Z-axis is internuclear axis) ?
(A) - Bond formation
(B) - Bond formation
(C) - Bond formation
(D) - Bond formation
(E) - Bond formation
(F) - Bond formation
1. A, B, C
2. C, F
3. B, E
4. B, C, D

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.






