Ideal gas is taken through cycle as shown in the figure. If net heat supplied to the gas in the cycle is 15 J, the work done by the gas in the process is
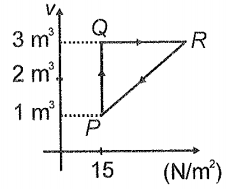
1. 5J
2. -15 J
3. -20 J
4. -12 J

Internal energy remains the same throughout the process along the path in
1 Adiabatic process
2 Isothermal process
3 Cyclic process
4 Both (2) & (3)
The cyclic process of \(2\) moles of diatomic gas is shown in the figure. Which of the following statements is correct?

| 1. | The process \(BC\) is an isothermal compression. |
| 2. | The work done in the process \(CA\) is \(4 R T_0\) |
| 3. | The work done in the process \(AB\) is zero. |
| 4. | All of these |
Without external heat work done possible in
1 Adiabatic process
2 Isochoric process
3 Isothermal process
4 Isobaric process
The PV diagram of an ideal gas is shown in the figure. The work done by the gas in the process is given by:
| 1. | \(\frac{9}{2}P_0V_0\) | 2. | \(\frac{15}{2}P_0V_0\) |
| 3. | \(\frac{13}{2}P_0V_0\) | 4. | \(\frac{3}{2}P_0V_0\) |
Given below are two statements:
| Statement I: | Zeroth law of thermodynamics explains the concept of thermal energy. |
| Statement II: | Thermal energy is independent of temperature. |
| 1. | Statement I is False but Statement II is True. |
| 2. | Both Statement I and Statement II are True. |
| 3. | Both Statement I and Statement II are False. |
| 4. | Statement I is true but Statement II is False. |
Which of the following is true for the molar heat capacity of an ideal gas?
| 1. | It cannot be negative. |
| 2. | It has only two values \(\left(C_P \text { and } C_V\right)\). |
| 3. | It can have any value. |
| 4. | It cannot be zero. |
An ideal gas undergoes a cyclic process as shown in the graph between pressure \((P)\) and temperature \((T).\) The part of the graph which represents the absorption of heat from the surrounding are:
1. \(\text{AB, CD}\)
2. \(\text{DA, BC}\)
3. \(\text{DA, AB}\)
4. \(\text{BC, AB} \)
During an adiabatic process, the pressure of a gas is found to be proportional to the fourth power of its absolute temperature. The ideal gas would be :
1.
2. He
3. The mixture of and He
4.
The pressure of a monoatomic gas increases linearly from N/m2 to N/m2 when its volume increases from 0.2 m3 to 0.5 m3. The molar heat capacity of the gas is:
[R = 8.31 J/mol k]
1. 20.1 J/molK
2. 17.14 J/molK
3. 18.14 J/molK
4. 20.14 J/molK








