The magnitude of the acceleration of the electron in the nth orbit of the hydrogen atom is and that of singly ionized helium atom is . The ratio is:
1. 1:8
2. 1:4
3. 1:2
4. Dependent on n

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
A hypothetical atom has 3 energy levels: the ground level, 2.00 eV, and 5.00 eV above the ground level. Which frequency of the spectral lines cannot be emitted when excited?
1.
2.
3.
4.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Which of the following statements is inconsistent with the Bohr model of the atom?
1. Energy levels of the electron are stable and discrete.
2. An electron emits or absorbs radiation only when making a transition from one energy level to another.
3. To jump from a lower energy to a higher energy orbit, an electron must absorb a photon of precisely the right frequency such that the photon's energy equals the energy difference between the two orbits.
4. To jump from a higher energy to a lower energy orbit, an electron absorbs a photon of a frequency such that the photon's energy is exactly the energy difference between the two orbits.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
The figure below illustrates an electron with an initial energy of -10 eV moving from point A to point B. What change accompanies the movement of the electron?
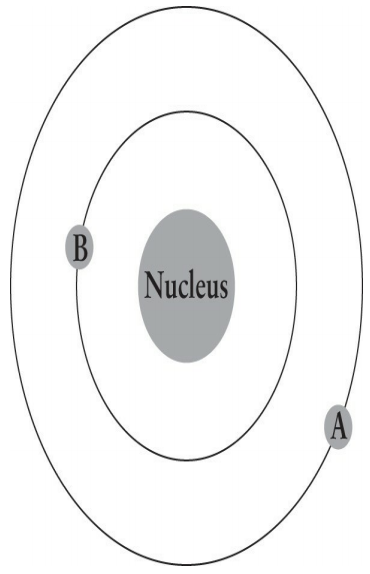
1. Absorption of a photon
2. Emission of a photon
3. The decrease in the atom's work function
4. Increase in the atom's total energy

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Two H atoms in the ground state collide inelastically. The maximum amount by which their combined kinetic energy is reduced -
1. 10.20 eV
2. 20.40 eV
3. 13.6 eV
4. 27.2 eV

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
The wavenumber of the energy emitted when an electron comes from the fourth orbit to the second orbit in hydrogen is 20, 397 . The wavenumber of the energy for the same transition in is-
1. 5,099
2. 20,497
3. 14400
4. 81,588

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
In the Bohr model of the hydrogen atom, let R, V and E represent the radius of the orbit, the speed of electron and the total energy of the electron respectively. Which of the following quantities is proportional to quantum number n?
1.
2.
3. RE
4. VR

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
The ratio of the energies of the hydrogen atom in its first to second excited states is:
1. 9/4
2. 4/1
3. 8/1
4. 1/8

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
If the shortest wavelength in the Lyman series is 911.6 , the longest wavelength in the same series will be:
1. 1600
2. 2430
3. 1215
4.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Hydrogen atoms are excited from the ground state to the principal quantum number 4. Then, the number of spectral lines observed will be:
1. 3
2. 6
3. 5
4. 2






