The threshold frequency of sodium for the photoelectric effect corresponds to yellow light. What happens when the bright yellow light is shone on the sodium metal?
1. No electrons are released from the metal.
2. The kinetic energy of the electrons increases.
3. The number of electrons ejected increases.
4. The threshold frequency of sodium metal increases.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
If the work function of a metal is and a ray of electromagnetic radiation with a frequency of is incident on the metal, what will be the speed of the electrons ejected from the metal?
1.
2.
3.
4.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
All of the following statements about the photoelectric effect are true Except:
1. the intensity Of the light beam does not affect the photocurrent.
2. the kinetic energies of the emitted electrons do not depend on the light intensity.
3. a weak beam Of light Of frequency greater than the threshold frequency yields more current than an intense beam of light of frequency lower than the threshold frequency.
4. for the light of a given frequency, the kinetic energy of emitted electrons increases as the value of the work function decreases.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Let be respectively the number of photons emitted by a red bulb and a blue bulb of equal power in a given time.
1.
2.
3.
4. data insufficient

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
When a photon of light collides with a metal surface, the number of electrons, (if any) coming out is:
1. only one
2. only two
3. infinite
4. depends upon factors

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
A point source of light is used in the photoelectric effect. If the source is removed farther from the emitting metal, the stopping potential:
1. will increase
2. will decrease
3. will remain constant
4. will either increase or decrease

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
What is the de-Broglie wavelength of a nitrogen molecule in air at 300 K? Assume that the molecule is moving with the root-mean-square speed of molecules at this temperature. ( Atomic mass of nitrogen = 14.0076 u)
1 0.01 nm
2 0.09 nm
3 0.03 nm
4 0.2 nm

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
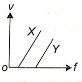
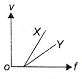
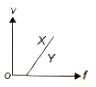
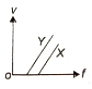
In a photoelectric experiment, electrons are ejected from metals X and Y by the light of intensity I and frequency f. The potential difference V required to stop the electrons is measured for various frequencies. If Y has a greater work function than X; which one of the following graphs best illustrates the expected results?
1. 
2. 
3. 
4. 

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
An electron with the initial kinetic energy of 100 eV is accelerated through a potential difference of 50 V. Now the de-Broglie wavelength of electron becomes-
1. 1
2.
3.
4. 12.27

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
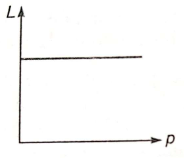
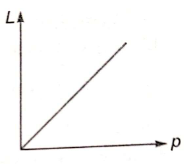
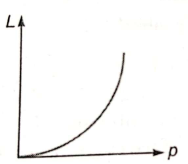
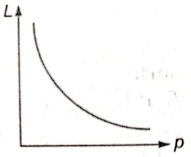
The de-Broglie wavelength L associated with an elementary particle of linear momentum p is best represented by the graph:
1. 
2. 
3. 
4. 

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.






