Oxidized form of enzyme catalase (structure A); prepared by the reaction of [Fe(P)]+ (P=porphyrin) with H2O2 has a green colour because of
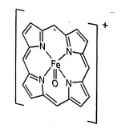
1. Oxidation state of iron changed from FeIII to FeIV.
2. Porphyrin ring is oxidized by one electron.
3. transition appears in the visible region
4. FeIV is coordinated with anionic tyrosinate ligand in axial position.

The cooperative binding of O2 in hemogoblin is due to
1. a decrease in size of iron followed by changes in the protein conformation
2. an increase in size of iron followed by changes in the protein conformation
3. a decrease in size of iron that is NOT accompanied by the protein conformational changes
4. an increase in size of iron that is NOT accompanied by the protein conformational changes
Molybdoenzymes can both oxidize as well as reduce the substrates, because
1. Mo(VI) is more stable than Mo(IV)
2. Mo(IV) can transfer oxygen atom to the substrate and Mo(VI) can abstract oxygen atom from the substrate
3. Conversion of Mo(VI) to Mo(IV) is not favoured
4. Mo(VI) can transfer oxygen atom to the substrate and Mo(IV) can abstract oxygen atom from the substrate
The metal present at the active site of the protein carboxypeptidase A is
1. Zinc
2. molybdenum
3. magnesium
4. Cobalt
The red colour of oxyhaemoglobin is mainly due to the
1. d-d transition
2. metal to ligand charge transfer transition
3. ligand to metal charge transfer transition
4. intraligand transition
Hemoglobin is an oxygen carrying protein. The correct statement about oxyhemoglobin is that
1. the metal is low-spin in +3 oxidation state while dioxygen is in form
2. the metal is high spin in +3 oxidation state while dioxygen is in form
3. the metal is low spin in +3 oxidation state while dioxygen is in neutral form
4. the metal is high spin in +3 oxidation state while dioxygen is in neutral form
Oxymyoglobin Mb(O2) and oxyhaemogolbin Hb(O2)4, respectively, are
1. Paramagnetic and paramagnetic
2. Diamagnetic and diamagnetic
3. Paramagnetic and diamagnetic
4. Diamagnetic and paramagnetic
The extent of electron conjugation in macrocyclic rings (i) heme, (ii) coenzyme B12 and (iii) chlorophyl follows the order
1. (i)>(iii)>(ii)
2. (i)>(ii)>(iii)
3. (iii)>(i)>(ii)
4. (ii)(i)>(iii)
Patients suffering from Wilson's disease have
1. Low level of Cu-Zn superoxide dismutase
2. High level of Cu-Zn superoxide dismutase
3. Low level of copper-storage protein, ceruloplasmin
4. High level of copper storage protein ceruloplasmin
Under physiological condition, oxygen is binding to deoxyhemoglobin the binding curve and its pH dependence, respectively are
1. Sigmoidal
2. hyperbolic
3. pH dependent
4. pH independent






