Which order for basic character of amine is correct for following compounds?

1. 3>1>2>5>4 2. 3>2>1>5>4
3. 3>1>2>4>5 4. 3>2>1>4>5

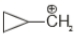
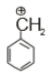
Which of the following is the most stable carbocation
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
1. 
2. 
3.
4.
What is the decreasing order of acidity for the compounds given below?

| 1. | III>IV>I>II | 2. | I>IV>III>II |
| 3. | II>I>III>IV | 4. | IV>III>I>II |
The correct increasing order of carbon-carbon bond length in the given compounds is:
1. III<II<I<IV
2. IV<I<II<III
3. I<II<III<IV
4. I<IV<III<II
In the following compounds,
The order of basicity is
1. IV>I>III>II
2. III>I>IV>II
3. II>I>III>IV
4. I>III>II>IV
Which of the following happens to be the carbanion with highest stability?
The IUPAC name of the following compounds is:

1. 1-Bromocyclohexane carboxylic acid
2. 3-Bromocyclohexanoic acid
3. 3-Bromoheptanoic acid
4. 3-Bromocyclohexanecarboxylic acid
Trans-2-butene on bromination using CS2 gives which dibromoderivative
1. (R), (R)-2,3-dibromobutane
2. (S),(S)-2,3-dibromobutane
3. Racemic-2,3-dibromobutane
4. Meso-2,3-dibromobutane
Which of the following carbocations is expected to be most stable?
1.
2.
3.
4.
A group that deactivates the benzene ring towards electrophilic substitution but which directs the incoming group principally to the o- and p-position is.
1. -NH2
2. -Cl
3. -NO2
4. -C2H5













