The reaction of HBr with propene in the presence of peroxide gives:
1. 3-bromopropane
2. Allyl bromide
3. n-propyl bromide
4. Isopropyl bromide
Using anhydrous AlCl3 as a catalyst, one of the following reaction that produces ethylbenzene (PhEt) is:
1.
2.
3.
4.
The molecular formula of diphenylmethane is C13H12.
The number of structural isomers possible when one of the hydrogens is replaced by a chlorine atom is:
| 1. | 4 | 2. | 8 |
| 3. | 7 | 4. | 6 |
On ozonolysis, the alkene that gives the following product is:
| 1. | |
| 2. | \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}=\mathrm{CHCH}_2 \mathrm{CH}_3\) |
| 3. | \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}=\mathrm{CHCH}_3\) |
| 4. |  |
A compound possessing optical isomerism provided it has the molecular formula C7H16, would be:
| 1. | 2,3-Dimethyl pentane | 2. | 2,2-Dimethyl butane |
| 3. | 2-Methyl hexane | 4. | None of the above |
The compound 
1. CH3COCH3
2. CH3COCH3 + CH3COOH
3. CH3COCH3 + CH3CHO
4. CH3CHO + CO2
Among the following, the correct example of a free-radical substitution reaction is:
| 1. | 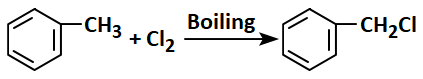 |
| 2. |  |
| 3. |  |
| 4. |  |
An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3-one.
The IUPAC name of ‘A’ is :
1. 3-Ethylpent-2-ene
2. 3-Ethylpent-2- yne
3. 2-Ethylpent-3-ene
4. 3-Ethylpent-4-yne
An alkene ‘A’ contains 3 C – Cσ bonds, 8 C – H σ bonds, and 1 C – C π bond,
‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. The IUPAC name of ‘A’ is:
| 1. | Prop-2-yne | 2. | But-2-ene |
| 3. | But-2-yne | 4. | Prop-2-ene |
Propanal and pentane-3-one are the products of ozonolysis of an alkene. The structural formula of the alkene is :
| 1. | 2. | ||
| 3. | 4. |  |







