Ortho nitrophenol is more acidic than ortho methoxy phenol because:
1.
The NO2-group increases the electron density in phenol while the methoxy group decreases the electron density in phenol
2.
The nitro-group is an electron-withdrawing group while the methoxy group is an electron-releasing group
3.
The methoxy-group is an electron-withdrawing group while the nitro group is an electron-releasing group
4.
None of the above
Preparation of ethers by 2 or 3-degree alcohols in an acidic medium is not a suitable method because:
| 1. | In case of secondary or tertiary alcohols, ketone is obtained as a product. |
| 2. | In case of secondary or tertiary alcohols, aldehyde is obtained as a product. |
| 3. | In case of secondary or tertiary alcohols, alkene is obtained as a product. |
| 4. | In case of primary alcohols, alkene is obtained as a product. |
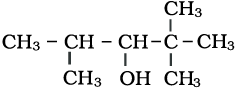
The IUPAC name of the above compound is:
1. 2, 2, 2-Trimethylpentan-3-ol
2. 2, 2, 4-Trimethylpentan-3-ol
3. 2, 2, 3-Trimethylpentan-3-ol
4. 1, 1, 3-Trimethylpentan-3-ol
The IUPAC name of the above compound is -
1. 2-Methylbutan-1-ol
2. 2-Methylbutan-2-ol
3. 1-Methylbutan-2-ol
4. 1-Methylbutan-1-ol
The IUPAC name of the below compound is:

1. 3,4-Dimethylhexane –1,3,5-triol
2. 3,5-Dimethylhexane –1,1,5-triol
3. 3,5-Dimethylhexane –1,3,5-triol
4. 3,1-Dimethylhexane –1,3,5-triol
The IUPAC name of
1. 3,3-Diethylphenol
2. 2,2-Diethylphenol
3. 1,3-Diethylphenol
4. 2,3-Diethylphenol

The IUPAC name of the above compound is:
| 1. | 4 – Ethoxypropane | 2. | 3 – Ethoxypropane |
| 3. | 1 – Ethoxypropane | 4. | 2 – Ethoxypropane |
The IUPAC name of the following compound is:

1. 1-Ethoxy-3-methylpentane
2. 3-Ethoxy-1-methylpentane
3. 2-Ethoxy-3-methylpentane
4. 3-Ethoxy-2-methylpentane
The IUPAC name of the following compound is -

1. Cyclohexane ethanol
2. Cyclohexane methanol
3. Cyclohexyl ethanol
4. Cyclohexyl methanol

The structure and name of intermediate compound is -
| 1. |  Cumene hydroperoxide |
| 2. |  Cumene hydroperoxide |
| 3. |  Cumene hydroperoxide |
| 4. | None of these |








