The significance of the octet rule is:
1. To determine the stability of atoms.
2. To find the hybridization of elements.
3. To calculate the number of lone pairs.
4. None of the above.
1. To determine the stability of atoms.
2. To find the hybridization of elements.
3. To calculate the number of lone pairs.
4. None of the above.
The incorrect statement among the following is:
| 1. | The sigma bond forms via head-on overlap and the pie bond forms via sidewise overlapping of orbitals. |
| 2. | s and p orbitals combine to form a sigma bond as well as a pie bond. |
| 3. | Hybrid orbitals form sigma bonds only. |
| 4. | Sigma bonds are stronger than pie bonds. |
The correct Lewis structure of acetic acid is:
| 1. |  |
2. |  |
| 3. |  |
4. | None of the above. |
H2 molecule on the basis of valence bond theory is formed due to:
1. Attractive forces being more than the repulsive forces.
2. Attractive forces being less than the repulsive forces.
3. No force of attraction.
4. Only repulsive force.
Hybridization of C1 and C2 in the structure given below are
1.
2.
3.
4.
Consider the following structure.
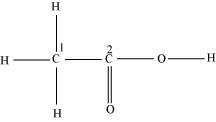
The hybridization of C1, and C2 carbon in the structure are -
1. = , =
2. = , =
3. = , =
4. None of the above
The number of resonating structures exhibited by \(CO^{2-}_3\) ion are :
| 1. | 2 | 2. | 3 |
| 3. | 4 | 4. | 5 |
The condition(s) required for the linear combination of atomic orbital is :
| 1. | Combining atomic orbitals must have the same or nearly the same energy. |
| 2. | Combining atomic orbitals must have proper orientations to ensure that the overlap is maximum. |
| 3. | Both 1 and 2 |
| 4. | None of the above |
The following graph captures potential energy on the y-axis for hydrogen gas formation as a function of the internuclear distance on the x-axis:
The bond energy of H2 can be represented by:
| 1. | (c – a) | 2. | (b – a) |
| 3. | (c-a)/2 | 4. | (b-a)/2 |
BF3 is a planar and an electron deficient compound. Hybridization and number of electrons around the central atom, respectively are:
1. sp2 and 6
2. sp2 and 8
3. sp3 and 4
4. sp3 and 6








