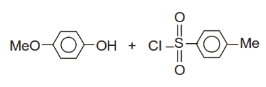The slowest step of Cannizarro's reaction is
1. Attack of nucleophilic
2. Hydride shift
3. Formation of anion
4. Transfer of proton
Subtopic: Isomers & Reaction Mechanism |
54%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Which acid deravative is most reactive towards nucleophilic substitution reaction?
1. CH3COCl
2. (CH3CO)2O
3. CH3COOC2H5
4. CH3CONH2
Subtopic: Acid Derivatives - Preparation, Properties & Uses |
71%
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
For the following reactions sequence
The structure consistent with X and Y are:
| 'Y' | 'X' | |
| (1) |  |
 |
| (2) |  |
 |
| (3) |  |
 |
| (4) |  |
 |
Subtopic: Carboxylic Acids: Preparation & Properties |
62%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
2–Phenylcycloprop–2–en–1–one is allowed to react with phenylmagnesium bromide and the reaction mixture is hydrolyzed with perchloric acid. The product formed is
| 1. |  |
| 2. |  |
| 3. |  |
| 4. | 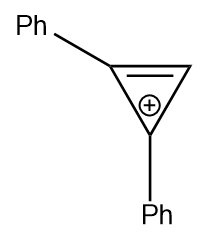 |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints

(1) 3, 2
(2) 3, 3
(3) 4, 2
(4) 4, 3
Subtopic: Aldehydes & Ketones: Preparation & Properties |
52%
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
The product is :
| (1) |  |
| (2) |  |
| (3) |  |
| (4) | None |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
52%
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
Major product is
| (1) |  |
| (2) |  |
| (3) |  |
| (4) | None of these |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints