The correct order of relative rates of hydrogenation of alkenes is:
1. Ethylene > propene > 2-butene > 2-methyl-2-butene
2. 2-methyl-2-butene > 2-butene > Propene > Ethylene
3. 2-butene > propene > ethylene > 2-methyl-2-butene
4. Propene > 2-butene > ethylene > 2-methyl-2-butene

 Products formed are
Products formed are
(A) 
(C) 
| (A) |  |
(B) |  |
| (C) |  |
(D) |  |
1. (A) and (B) only 2. (A) and (C) only
3. (B) and (C) only 4. (A), (B), (C) and (D)
An unsaturated hydrocarbon on complete hydrogenation gives 1-isopropyl-3 methylcyclohexane, after ozonolysis it gives one mole of formaldehyde, one mole of acetone and one mole of 2,4-Dioxohexanedial. The possible structure\s of the hydrocarbon may be
1. 
2. 
3. 
4.
Which one of the following reactions is not possible?
1. CH3COONa + HCI → CH3COOH + NaCI
2. CH3–SO3H + → CH3SONa +
3. + PhONa → PhOH +
4. + NaNH2→
+ NH3
Observe the following sequence of reactions.

The product R is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
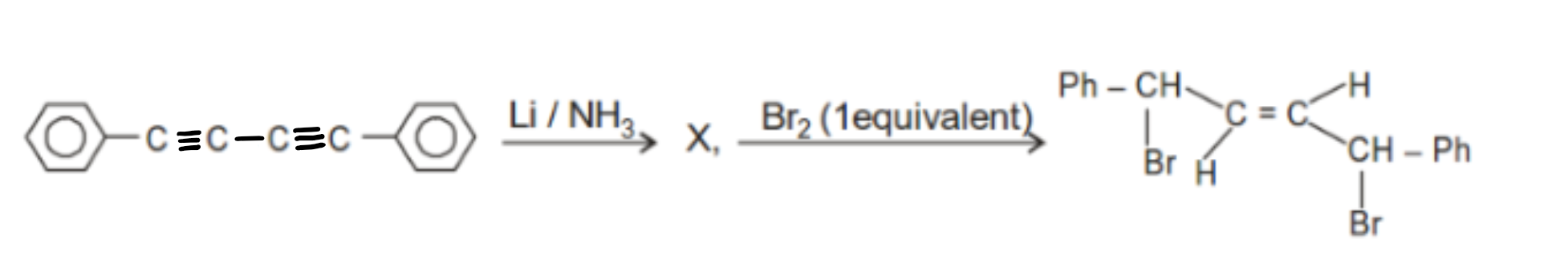
'X' is :
| (1) |  |
| (2) |  |
| (3) | Both |
| (4) | None of these |
Which of the following is a correct statement regarding the relative acidic character of cyclopropane and propane?
1. Cyclopropane is more acidic than propane
2. Propane is more acidic than cyclopropane
3. Both are equally acidic
4. Bothe are neutral
No . of products and No. of fractions are respectively
(1) 6, 5
(2) 6, 4
(3) 5, 4
(4) 6, 3
Consider the following reaction
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
In the following series of reactions the major products P and S are respectively.
1.
2.
3.
4.














