(CH3)3CCI + (CH3)3C K+ → Product
1. SN Product will be more
2. E2 Product will be more
3. Both will be the same
4. None of the above
Neopentyl iodide is treated with aq. AgNO3 solution, a yellow precipitate is formed along with another compound which is
1. (CH3)3CCH2ONO2
2.
3. (CH3)3CCH2OH
4.
The major end product of the following reaction is
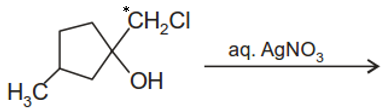
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Z
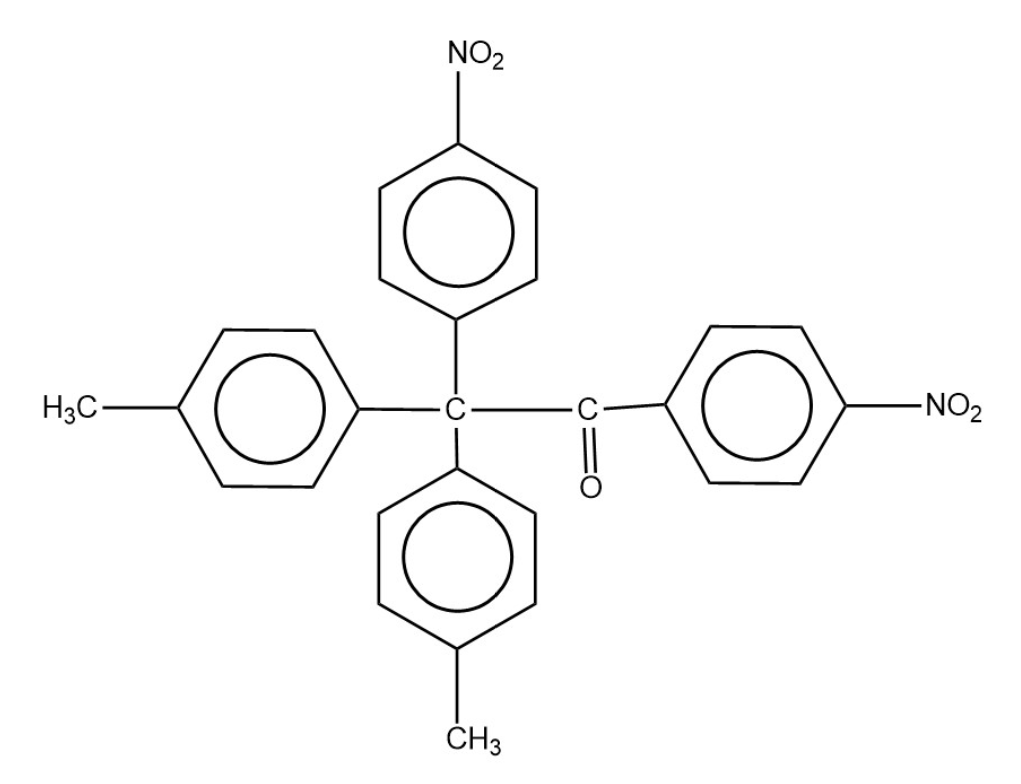
The product of following reacting is
| (1) |  |
| (2) |  |
| (3) |  |
| (4) |  |
The product in the given reaction is
| (1) |  |
| (2) |  |
| (3) |  |
| (4) |  |
Conversion of cyclohexene to cyclohexanol can be conveniently achieved by:
1. NaOH + H2O
2. Br2-H2O
3. Hydroboration, oxidation
4. Hydroboration hydrolysis
Bromination of (E)-2-butenedioic acid gives
(1) (2R, 3S)-2, 3-dibromosuccinic acid
(2) (2R, 3R)-2, 3-dibroniosuccinic acid
(3) a mixture of (2R, 3R) and (2S, 3S)-2, 3-dibromosuccinic acid
(4) (2S, 3S)-2, 3-dibromosuccinic acid

 Product, Product is
Product, Product is


 The product is –
The product is –