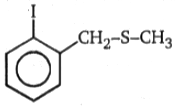
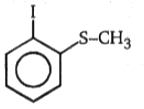
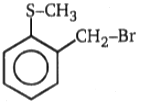
Which of the following alkyl halide undergo rearrangement in SN1 reaction?
1.
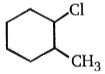
2. 
3. 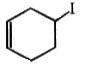
4. All of these


| 1. |  |
2. |  |
| 3. |  |
| 1. | 1 > 2 > 3 | 2. | 2 > 3 > 1 |
| 3. | 2 > 1 > 3 | 4. | 3 > 2 > 1 |
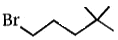
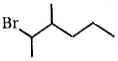
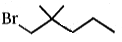
Which compound undergoes nucleophilic substitution with NaCN at the fastest rate ?
1. 
2. 
3. 
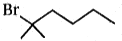
4. 
Rank the following in order of decreasing rate of solvolysis with aqueous ethanol (fastest slowest)
| a. |  |
b. |  |
| c. |   |
1. b>a>c
2. a>b>c
3. b>c>a
4. a>c>b
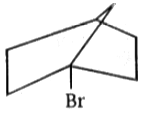
Which of the following reactant will not favour SN2 nucleophilic substitution reaction?
1. 
2. Ph-Br
3.
4. All of the above
What is the major product of the following reaction?
1.
2.
3.
4.
SN1 and SN2 products are the same with (excluding stereoisomer):-
1. 
2. 
3. 
4.
Consider the nucleophilic attacks given below. Select in each pair that shows the greater SN2 reaction rate.
(A) 
(B)
(C) 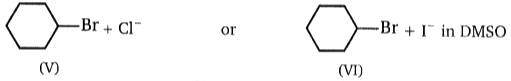
(D) 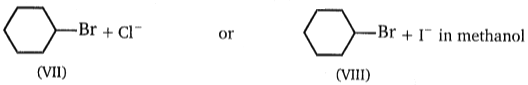
A B C D
1. II IV VI VIII
2. II III V VIII
3. I III V VIII
4. I III V VII
Addition of KI accelerates the hydrolysis of primary alkyl halides because:
1. KI is soluble in organic solvents
2. the iodide ion is a weak base and a poor leaving group
3. the iodide ion is a strong base
4. the iodide ion is a powerful nucleophile as well as a good leaving group



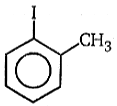 (A)(B), Product (B) is:
(A)(B), Product (B) is: