p-Toluidine reacts with benzene diazonium chloride to form compound, which on boiling with aq. H2SO4 give .......... products:
(1) 3
(2) 2
(3) 4
(4) 5
(1) 3
(2) 2
(3) 4
(4) 5
Subtopic: Urea & Nitro Compound |
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
Product (C) in the above reaction is:
1.
2.
3.
4.
Subtopic: Urea & Nitro Compound |
76%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
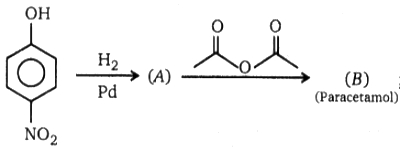 ; Product (B) of this reaction is :
; Product (B) of this reaction is :
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
Cyclopentadiene is much more acidic than cyclopentane, because:
| 1. | Cyclopentadiene has conjugated double bonds. |
| 2. | Cyclopentadiene has both sp2 and sp3 hybridized carbon atoms. |
| 3. | Cyclopentadiene is a strain-free cyclic system. |
| 4. | Cyclopentadienyl anion ion, the conjugate base of cyclopentadiene, is an aromatic species and hence has higher stability. |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
67%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
The compounds among the following that can be generated by Friedel craft acylation is/are -
| I. |  |
| II. |  |
| III. |  |
| IV. |  |
| 1. | II, III and IV | 2. | I, III and IV |
| 3. | I and II | 4. | II and III |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
74%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
The product B in the above-mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
56%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
The major product obtained in the given reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
88%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
 |
The labelled carbon goes with:
Subtopic: Carboxylic Acids: Preparation & Properties |
67%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
The deactivating but ortho-para directing benzene ring substituent among the following is-
1. -N=O
2. -OCH3
3. -COCH3
4. -NO2
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch















