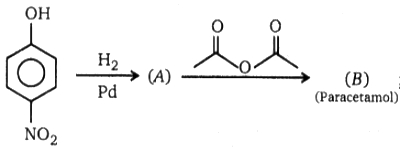
 ; Product (B) of this reaction is :
; Product (B) of this reaction is :
1.

2.

3.

4.

 ; Product (B) of this reaction is :
; Product (B) of this reaction is :



Cyclopentadiene is much more acidic than cyclopentane, because:
| 1. | Cyclopentadiene has conjugated double bonds. |
| 2. | Cyclopentadiene has both sp2 and sp3 hybridized carbon atoms. |
| 3. | Cyclopentadiene is a strain-free cyclic system. |
| 4. | Cyclopentadienyl anion ion, the conjugate base of cyclopentadiene, is an aromatic species and hence has higher stability. |
The compounds among the following that can be generated by Friedel craft acylation is/are -
| I. |  |
| II. |  |
| III. |  |
| IV. |  |
| 1. | II, III and IV | 2. | I, III and IV |
| 3. | I and II | 4. | II and III |
The product B in the above-mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The major product obtained in the given reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The deactivating but ortho-para directing benzene ring substituent among the following is-
1. -N=O
2. -OCH3
3. -COCH3
4. -NO2
Which of the following organic chlorides will not give a Friedel-Craft alkylation product when heated with benzene and AlCl3/FeCl3?
| 1. | (CH3)3CCl | 2. | CH2=CHCH2Cl |
| 3. | CH3CH2Cl | 4. | CH2=CHCl |
Which of the following is aromatic?
1.
2. 
3.
4. 
The compound X in the above reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Give a simple test to differentiate cyclohexane and cyclohexene :
1. Br2/H2O
2. Baeyer's reagent
3. Tollen's reagent
4. Both (1) and (2)











