The IUPAC name of the given compound is:

1. Ethanoic propanoic anhydride
2. Propanoic ethanoic anhydride
3. 1-Ethanoyloxypropanone
4. 3-Ethanoyloxypropan-3-one

The IUPAC name of the given-below compound is:
1. Butane-2,3-dial
2. Butane-1,3-dione
3. Butane-2,3-dione
4. 1, 2-Dimethylethanedione
The IUPAC name of 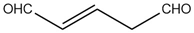 is:
is:
(1) propene-1, 3-dial
(2) Propene-1, 3-dicarbaldehyde
(3) Pent-3-ene-1, 5-dial
(4) Pent-2-ene-1, 5-dial
| IUPAC name of the following compound is: |
\(CH_3-CH-CH_2-O-C_2H_5\\ ~~~~~~~~~~~~~~|\\ ~~~~~~~~~~~~~OH\)
1. 1-Ethoxypropan-2-ol
2. 3-Ethoxypropan-2-ol
3. 1-Ethoxy-2-hydroxypropane
4. None of these
The IUPAC name of 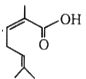 is:
is:
1. 2, 6-Dimethylhepta-2, 5-dienoic acid
2. 3, 7-Dimethylhepta-2, 5-dienoic acid
3. 1-Hydroxy-2, 6-dimethylhepta-2, 5-dienone
4. None of the above
The silver sulphate solution is used to separate:
1. nitrate and bromide
2. nitrate and chlorate
3. bromide and iodide
4. nitrate and nitrite
The most stable carbocation among the following is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The electromeric effect in organic compounds is a
1. Temporary effect
2. Permanent effect
3. Electromeric effect is only observed in inorganic molecules
4. None of the above
The stability of 2,3-dimethyl but-2-ene is more than 2-butene. This can be explained in terms of:
1. resonance
2. hyperconjugation
3. electromeric effect
4. inductive effect
(CH3)4N+ is neither an electrophile, nor a nucleophile because it:
1. does not have electron pair for donation as well as cannot attract electron pair
2. neither has electron pair available for donation nor can accommodate electron since all shells of N are fully occupied.
3. can act as Lewis acid and base
4. none of the above







