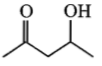
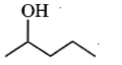
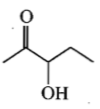
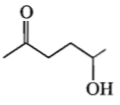
Which one of the following will most readily be dehydrated in acidic conditions?
1. 
2. 
3. 
4. 




1-propanol and 2-propanol can be best distinguished by:-
(1) oxidation with alkaline KMnO4 followed by reaction with Fehling's solution
(2) oxidation with acidic dichromate followed by reaction with Fehling’s solution
(3) oxidation by heating with copper followed by reaction with Fehling’s solution
(4) oxidation with conc. H2SO4 followed by reaction with Fehling’s solution
Phenyl magnesium bromide reacts with methanol to give a mixture of:-
1. Anisole and Mg(OH)Br
2. Benzene and Mg(OMe)Br
3. Toluene and Mg(OH)Br
4. Phenol and Mg(Me)Br
When wine is put in air it become sour due to:-
(1) oxidation of C2H5OH into CH3COOH
(2) bacteria
(3) virus
(4) formic acid formation
Which enzyme catalyzes the conversion of glucose to ethanol?
1. Zymase
2. Diastase
3. Maltase
4. Invertase
Denatured spirit is mainly used as a:
(1) good fuel
(2) drug
(3) solvent in preparing varnishes
(4) material in the preparation of oil
The reaction of ethanol with H2SO4 does not give:
1. C2H4
2. C2H5OC2H5
3. C2H2
4.C2H5HSO4
The products of combustion of an aliphatic thiol (RSH) at 298K are:-
(1) CO2(l), H2O(g)andSO2(g)
(2) CO2(g), H2O(g)andSO2(g)
(3) CO2(l), H2O(l)andSO2(g)
(4) CO2(g), H2O(l)andSO2(l)
Which reagent will convert propionic acid to propanol-1?
(1) KMnO4
(2) LiAlH4
(3) Cr2O3
(4) MnO2
A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was:
(1) HCHO
(2) CH3COCH3
(3) CH3COOH
(4) CH3OH






