The compound that does not react with sodium is-
1. CH3CHOHCH3
2. CH3 —O—CH3
3. CH3COOH
4. C2H5OH
An organic compound A reacts with sodium metal and forms B. On heating with conc. H2SO4, A gives diethyl ether. So A and B are:
1. C3H7OH and CH3ONa
2. CH3OH and CH3ONa
3. C4H9OH and C4H9ONa
4. C2H5OH and C2H5ONa
Phenol on treatment with dil. HNO3 at room temperature gives-
| 1. |  |
| 2. |  |
| 3. |  |
| 4. | All of the above |
Identify A and predict the type of reactions
1. 
2.
3.
4.
Identify A, X, Y, and Z:
1. A-methoxymethane, X-ethanoic acid, Y-acetate ion, Z-hydrazine
2. A- methoxymethane, X-ethanol, Y-ethanoic acid, Z- semicarbazide
3. A-Acetaldehyde, X-ethanol, Y-but-2-en-1-ol, Z- semicarbazone
4. A-ethanol, X-acetaldehyde, Y-butanone, Z-hydrazone
Which one is the most acidic compound ?
The reaction
can be classified as
1. Alcohol formation reaction
2. Dehydration reaction
3. Williamson alcohol synthesis reaction
4. Williamson ether synthesis reaction
Reaction of phenol with chloroform in the presence of dilute sodium hydroxide finally
introduces, which one of the following functional group?
1. -CH2Cl
2. -COOH
3. -CHCl2
4. -CHO
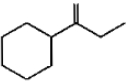
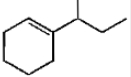
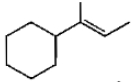
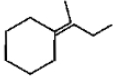
Which of the following is not the product of dehydration of
1. 
2. 
3. 
4. 
Among the following ethers, the one that will produce methyl alcohol on treatment with hot concentrated HI is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |











