An aldehyde which undergoes Cannizzaro's reaction and reduces Schiff's reagent but does not reduce Fehling's solution is:
(1) CH3CHO
(2) HCHO
(3) C6H5CHO
(4) salicylaldehyde
b-hydroxy butyraldehyde is an example of:
(1) aldol
(2) diol
(3) hemiacetal
(4) acetal
A ketone reacted with C2H5MgBr reagent followed by hydrolysis gave a product which on dehydration gives an alken. The alkene on ozonolysis gave diethyl ketone and acetaldehyde. The ketone is:
(1) dimethyl ketone
(2) ethyl methyl ketone
(3) diethyl ketone
(4) ethyl propyl ketone
The reaction 
1.
2.
3.
4.
Treatement of propionaldehyde with dil. NaOH gives:
(1)
(2)
(3)
(4)
Which of the following will react with water ?
(1) CHCl3
(2) CCl3CHO
(3) CCl4
(4) CH2Cl.CH2Cl
Among the given compounds, the most susceptible to nucleophile attack at the carbonyl group is:
(1) MeCOCl
(2) MeCHO
(3) MeCOOMe
(4) MeCOOCOMe
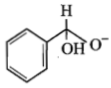
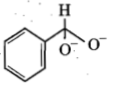
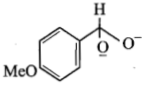
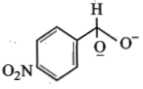
In a Cannizzaro's reaction, the intermediate that will be best hydride donor is:
1. 
2. 
3. 
4. 
In the Cannizzaro's reaction given below,
the slowest step is:
1. The attack of OH- at the carbonyl group
2. The transfer of hydride to the carbonyl group
3. The abstraction of proton from the carboxylic acid
4. the deprotonation of Ph-CH2OH
The aldol condensation of acetaldehyde results in hte formation of:
1.
2.
3.
4.










