A ketone reacted with C2H5MgBr reagent followed by hydrolysis gave a product which on dehydration gives an alken. The alkene on ozonolysis gave diethyl ketone and acetaldehyde. The ketone is:
(1) dimethyl ketone
(2) ethyl methyl ketone
(3) diethyl ketone
(4) ethyl propyl ketone
The reaction 
1.
2.
3.
4.
Treatement of propionaldehyde with dil. NaOH gives:
(1)
(2)
(3)
(4)
Which of the following will react with water ?
(1) CHCl3
(2) CCl3CHO
(3) CCl4
(4) CH2Cl.CH2Cl
Among the given compounds, the most susceptible to nucleophile attack at the carbonyl group is:
(1) MeCOCl
(2) MeCHO
(3) MeCOOMe
(4) MeCOOCOMe
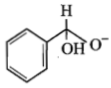
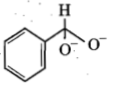
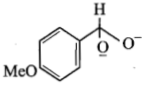
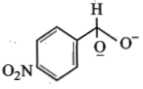
In a Cannizzaro's reaction, the intermediate that will be best hydride donor is:
1. 
2. 
3. 
4. 
In the Cannizzaro's reaction given below,
the slowest step is:
1. The attack of OH- at the carbonyl group
2. The transfer of hydride to the carbonyl group
3. The abstraction of proton from the carboxylic acid
4. the deprotonation of Ph-CH2OH
The aldol condensation of acetaldehyde results in hte formation of:
1.
2.
3.
4.
Wolff-Kishner's reaction is:
1. reduction of carbonyl compound into hydrocarbons
2. reduction of carbonyl compound into alcohols
3. reduction of nitrobenzene into aniline
4. reduction of carbohydrates to alcohol
The correct order of reactivity of PhMgBr with,
(1) I>II>III
(2) III>II>I
(3) II>III>I
(4) II>I>III










