Biuret test is not given by:
(1) proteins
(2) carbohydrates
(3) polypeptides
(4) urea
An aromatic amine (A) reacts with alcoholic potash and another compound (Y), producing a foul-smelling gas with the formula \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{NC}\).
The compound (Y) is obtained by reacting compound (Z) with chlorine (\(\mathrm{Cl}_2\)) in the presence of slaked lime.
What is the identity of compound (Z)?
1. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2\)
2. \(\mathrm{CH}_3 \mathrm{OH}\)
3. \(\mathrm{CH}_3 \mathrm{CH}_2\mathrm{OH}\)
4. \(\mathrm{CHCl}_3\)
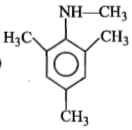
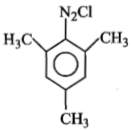
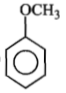
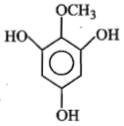
Identify 'Z' in the reaction given below;
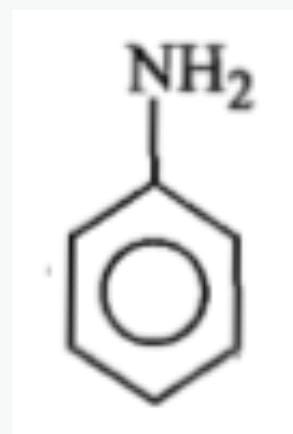
1. 
2. 
3. 
4. 
The product obtained on nitration followed by alkaline hydrolysis of acetylated aniline is:
1. o-Nitroacetanilide
2. o- and p-Nitroaniline
3. m-Nitroaniline
4. Acetanilide
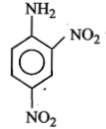
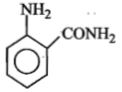
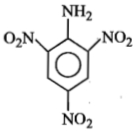
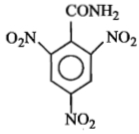
The formula of picramide is :
1. 
2. 
3. 
4. 
Pyridine possesses :
(1) aromatic nature
(2) unsaturated aliphatic nature
(3) alicyclic nature
(4) aliphatic nature
Ammoniacal solution of Ni(CN)2 reacts with C6H6 to produce a light violet coloured crystaline compound of the formula:
(1)
(2)
(3)
(4)
The diazonium salt 

1. HCl/CuCl
2. HNO2/Cu
3. C2H5OH
4. SnCl2/HCl
The reaction, is called:
1. Etard's reaction
2. Sandmeyer's reaction
3. Wurtz-Fitting reaction
4. Perkin's reaction
Choose the correct statement from the ones given below for two anilium in:
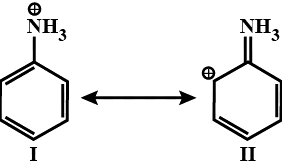
1. II is not an acceptable canonical structure because carbonium ions are less stable than ammonium ions
2. II is not an acceptable canonical structure because it is non-aromatic
3. II is not an acceptable canonical structure because the nitrogen has 10 valence electrons
4. II is an acceptable canonical structure






