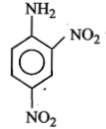
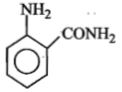
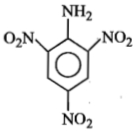
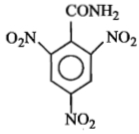
An aromatic amine (A) reacts with alcoholic potash and another compound (Y), producing a foul-smelling gas with the formula \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{NC}\).
The compound (Y) is obtained by reacting compound (Z) with chlorine (\(\mathrm{Cl}_2\)) in the presence of slaked lime.
What is the identity of compound (Z)?
1. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2\)
2. \(\mathrm{CH}_3 \mathrm{OH}\)
3. \(\mathrm{CH}_3 \mathrm{CH}_2\mathrm{OH}\)
4. \(\mathrm{CHCl}_3\)
The compound (Y) is obtained by reacting compound (Z) with chlorine (\(\mathrm{Cl}_2\)) in the presence of slaked lime.
What is the identity of compound (Z)?
1. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2\)
2. \(\mathrm{CH}_3 \mathrm{OH}\)
3. \(\mathrm{CH}_3 \mathrm{CH}_2\mathrm{OH}\)
4. \(\mathrm{CHCl}_3\)
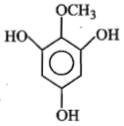
Identify 'Z' in the reaction given below;
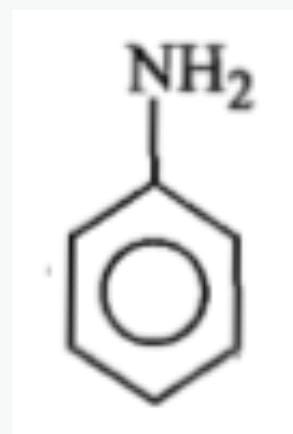
1. 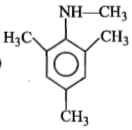
2. 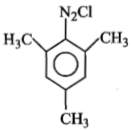
3. 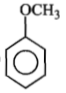
4. 
The product obtained on nitration followed by alkaline hydrolysis of acetylated aniline is:
1. o-Nitroacetanilide
2. o- and p-Nitroaniline
3. m-Nitroaniline
4. Acetanilide
Pyridine possesses :
(1) aromatic nature
(2) unsaturated aliphatic nature
(3) alicyclic nature
(4) aliphatic nature
Ammoniacal solution of Ni(CN)2 reacts with C6H6 to produce a light violet coloured crystaline compound of the formula:
(1)
(2)
(3)
(4)
The diazonium salt 

1. HCl/CuCl
2. HNO2/Cu
3. C2H5OH
4. SnCl2/HCl
The reaction, is called:
1. Etard's reaction
2. Sandmeyer's reaction
3. Wurtz-Fitting reaction
4. Perkin's reaction
Choose the correct statement from the ones given below for two anilium in:
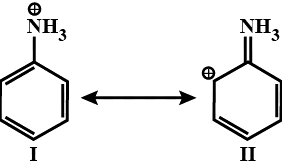
1. II is not an acceptable canonical structure because carbonium ions are less stable than ammonium ions
2. II is not an acceptable canonical structure because it is non-aromatic
3. II is not an acceptable canonical structure because the nitrogen has 10 valence electrons
4. II is an acceptable canonical structure
When nitrobenzene is treated with Br2 in presence of FeBr3, the major product formed is m-bromonitrobenzene. Statements which are related to obtain the m-isomer are:
(1) the relative electron density on meta carbon is more than that of ortho and para positions
(2) loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta position
(3) easier loss of H+ to regain aromaticity from the meta positions than from ortho and para positions
(4) none of the above