Consider the following bromides:

the correct order of SN1 reactivity is:
1. A>B>C
2. B>C>A
3. B>A>C
4. C>B>A

In an SN1 reaction on chiral centres there is
1. 100% racemisation
2. inversion more than retention leading to partial racemisation
3. 100% retention
4. 100% inversion
If X is halogen the correct order for SN2 reactivity is-
1. R2CHX > R3CX > RCH2X
2. RCH2X > R3CX > R2CHX
3. RCH2X > R2CHX > R3X
4. R3CX > R2CHX > RCH2X
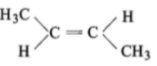
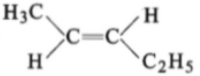
The compound which reacts with HBr obeying Markownikoff's rule is:
1.
2. 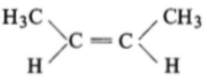
3. 
4. 
The formation of cyanohydrin from a ketone is an example of:
(1) electrophilic addition
(2) nucleophilic addition
(3) nucleophilic substitution
(4) electrophilic substitution
Which of the following is an example of the elimination reaction?
1. Chlorination of methane
2. Dehydration of ethanol
3. Nitration of benzene
4. Hydroxylation of ethylene
The elimination reaction among the following is:
1. CH3CH3+Cl2CH3CH2Cl+ HCl
2. CH3Cl+ KOH(aq.) CH3OH +KCl
3. CH2=CH2 +Br2 CH2BrCH2Br
4. C2H5Br+ KOH(alc.) C2H4 + KBr+H2O
SN1 mechanism for the reaction,
R-X+KOHROH+KX follow:
(1) carbocation mechanism
(2) carbanion mechanism
(3) free radical mechanism
(4) either of these
Which step is chain propagation step in the following mechanism?
1. (i)
2. (ii)
3. (iii)
4. (iv)
Which step is the chain termination step in the following mechanism?
1. (i)
2. (ii)
3. (iii)
4. (iv)






