In following reaction, find out X and Y.
1. X=2-Butyne ; Y=3-Hexyne
2. X=2-Butyne ; Y=2-Hexyne
3. X=1-Butyne ; Y=2-Hexyne
4. X=1-Butyne ; Y=3-Hexyne
An organic compound X having molecular formula C5H10O yields phenyl hydrazone and
gives negative response to the iodoform test and Tollen's test. It produces n-pentane on
reduction. X could be:
1. Pentenal
2. 2-Pentanone
3. 3-Pentanone
4. n-Amyl alcohol
Which of the following will not be soluble in sodium hydrogen carbonate?
1. 2, 4,6 - trinitrophenol
2. benzoic acid
3. o-nitrophenol
4. Benzenesulphonic acid
In a reaction,
A is
1. HgSO4/H2SO4
2. Cu2Cl2
3. H3PO2 and H2O
4. H+/H2O
In the following sequence of reactions
CH3-Br ABC
the end product C is:
1. Acetone
2. Methane
3. Acetaldehyde
4. Ethyl alcohol
CH3CHO and C6H5CH2CHO can be distinguished chemically by:
1. Benedict test
2. Iodoform test
3. Tollen's reagent test
4. Fehling solution test
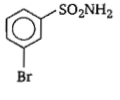
In a set of reactions, m-bromobenzoic acid gave a product D. Identify the product D.

1.
2.
3.
4. 
What is the product obtained in the following reaction?
Which one is a nucleophilic substitution reaction among the following?
| 1. |  |
| 2. |  |
| 3. | \(\mathrm{CH}_3 \mathrm{CHO}+\mathrm{HCN} \rightarrow \mathrm{CH}_3 \mathrm{CH}(\mathrm{OH}) \mathrm{CN}\) |
| 4. |  |
The reaction of toluene with Cl2 in presence of FeCl3 gives 'X' and reaction in presence of
light gives 'Y'. Thus, 'X' and 'Y' are
1. X = Benzal chloride, Y = o-chlorotoluene
2. X = m-chlorotoluene, Y= p-chlorotoluene
3. X = o-and p-chlorotoluene, Y=trichloromethyl benzene
4. X = Benzyl chloride, Y = m-chlorotoluene












