Reaction of phenol with chloroform in the presence of dilute sodium hydroxide finally
introduces, which one of the following functional group?
1. -CH2Cl
2. -COOH
3. -CHCl2
4. -CHO
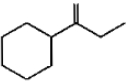
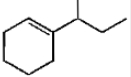
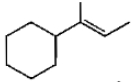
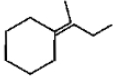
Which of the following is not the product of dehydration of
1. 
2. 
3. 
4. 
H2COH.CH2OH on heating with periodic acid gives :-
1. 2CO2
2. 2HCOOH
3. CHO-CHO
4.
Acetophenone when reacted with a base, C2H5ONa, yields a stable compound which has the structure
The major organic product in the reaction, CH3 — O — CH(CH3)2 + HI Product is :
(1) CH3OH+(CH3)2CHI
(2) ICH3OCH(CH3)2
(3) CH3O CI(CH3)2
(4) CH3I+(CH3)2CHOH
Identify A, X, Y and Z
(1) A-methoxymethane, X-ethanoic acid, Y-acetate ion, Z-hydrazine
(2) A- methoxymethane, X-ethanol, Y-ethanoic acid, Z- semicarbazide
(3) A-Acetaldehyde, X-ethanol, Y-but-2-en-1-ol, Z- semicarbazone
(4) A-ethanol, X-acetaldehyde, Y-butanone, Z-hydrazone
Which will form two oximes with NH2OH?
(1) CH3COCH3
(2) CH3CH2COCH3
(3) CH3CH2COCH2CH3
(4)
An organic compound A reacts with sodium metal and forms B. On heating with conc. H2SO4, A gives diethyl ether. So A and B are:
1. C3H7OH and CH3ONa
2. CH3OH and CH3ONa
3. C4H9OH and C4H9ONa
4. C2H5OH and C2H5ONa
Phenyl ethyl ether with concentrated hydrobromic acid on boiling yields:-
1. Phenol and ethyl bromide
2. Bromobenzene and ethanol
3. Phenol and ethane
4. Bromobenzene and ethane
A compound with molecular formula C4H10O3 is converted by the action of acetyl chloride to a compound with molar mass 190. The original compound has:
(1) one OH group
(2) two OH groups
(3) three OH groups
(4) no OH group











