Given is cyclohexanol (I), acetic acid (II), 2,4,6-trinitrophenol (III), and phenol (IV).
The order of decreasing acidic character will be
1. III > II > IV > I
2. II>III>I>IV
3. II > III > IV > I
4. III > IV > II > I
which one of the following has the most acidic nature?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The correct order of increasing reactivity of C-X bond towards nucleophile in the following
compounds is
1. I < II < IV < III
2. II < III < I < IV
3. IV < III < I < IV
4. III < II < I < IV
Among the given compounds, the most susceptible to nucleophilic attack at the carbonyl group is
1. CH3COOCH3
2. CH3CONH2
3. CH3COOCOCH3
4. CH3COCl
Which one is most reactive towards electrophilic reagent?
The IUPAC name of the compound having the formula CHC-CH=CH2 is
1. 3-butene-1-yne
2. 1-butyn-3-ene
3. but-1-yne-3-ene
4. 1-butene-3-yne
The relative reactivates of acyl compounds towards nucleophilic substitution are in the order of
(1) Acyl chloride > Acid anhydride > Ester > Amide
(2) Ester > Acyl Chloride > Amide > Acid anhydride
(3) Acid anhydride > amide > Ester > Acyl chloride
(4) Acyl chloride > Ester > Acid anhydride > Amide
Which one of the following is most reactive towards electrophillic attack?
(a)
(b)
(c)
(d)
A strong base can abstract an -hydrogen from
1. alkene
2.amine
3. ketone
4. alkane
The stability of carbanions in the following
(1)
(2) 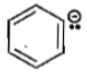
(3)
(4)
is in the order of
(a) (1)>(2)>(3)>(4)
(b) (2)>(3)>(4)>(1)
(c) (4)>(2)>(3)>(1)
(d) (1)>(3)>(2)>(4)












