Which of the following is most reactive towards electrophilic reagents?
1.

2.

3.

4.





The IUPAC name of the compound having the formula CHC-CH=CH2 is
1. 3-butene-1-yne
2. 1-butyn-3-ene
3. but-1-yne-3-ene
4. 1-butene-3-yne
The relative reactivates of acyl compounds towards nucleophilic substitution are in the order of
(1) Acyl chloride > Acid anhydride > Ester > Amide
(2) Ester > Acyl Chloride > Amide > Acid anhydride
(3) Acid anhydride > amide > Ester > Acyl chloride
(4) Acyl chloride > Ester > Acid anhydride > Amide
Which one of the following is most reactive towards electrophillic attack?
(a)
(b)
(c)
(d)
A strong base can abstract an -hydrogen from
1. alkene
2.amine
3. ketone
4. alkane
The stability of carbanions in the following
(1)
(2) 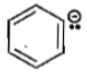
(3)
(4)
is in the order of
(a) (1)>(2)>(3)>(4)
(b) (2)>(3)>(4)>(1)
(c) (4)>(2)>(3)>(1)
(d) (1)>(3)>(2)>(4)
If there is no rotation of plane polarized light by a compound in a specific solvent, thought to be chiral,
it may mean that:
(1) the compound is certainly a chiral
(2) the compound is certainly meso
(3) there is no compound in the solvent
(4) the compound may be a racemic mixture
For the following :
(i) I-
(ii) Cl-
(iii) Br-
the increasing order of nucleophilicity would be :
1. I- < Br- < Cl-
2. Cl- < Br- < I-
3. I- < Cl- < Br-
4. Br- < Cl- < l-
Among the given compounds, the most susceptible to nucleophilic attack at the carbonyl group is
1. CH3COOCH3
2. CH3CONH2
3. CH3COOCOCH3
4. CH3COCl
The correct order of increasing reactivity of C-X bond towards nucleophile in the following
compounds is
1. I < II < IV < III
2. II < III < I < IV
3. IV < III < I < IV
4. III < II < I < IV











