In SN2 substitution reaction of the type
Which one of the following has the highest relative rate ?
(1)
(2)
(3)
(4)
Which one is the most reactive toward SN1 reaction?
1. C6H5CH(C6H5)Br
2. C6H5CH(CH3)Br
3. C6H5C(CH3)(C6H5)Br
4. C6H5CH2Br
The reaction of C6H5CH=CHCH3 with HBr produces
The compound that will react most readily with gaseous bromine has the formula ?
(1) C3H6
(2) C2H2
(3) C4H10
(4) C2H4
Consider the reaction
CH3CH2CH2Br + NaCN CH3CH2CH2CN + NaBr
This reaction will be the fastest in
1. ethanol
2. methanol
3. N,N'-dimethylformamide (DMF)
4. water
In the given reaction

the product P is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Which of the following can be used as the halide component for Friedel-Crafts reaction?
(1) Chlorobenzene
(2) Bromobenzene
(3) Chloroethene
(4) Isopropyl chloride
AgNO3(aq) gives yellow ppt. with:
(1) KIO3 (aq)
(2) KI (aq)
(3) CHI3
(4) CH2I2
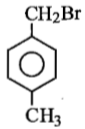
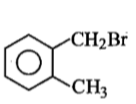
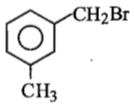
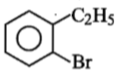
Compound (A), C8H9Br, gives a white precipitate when warmed with alcoholic AgNO3. Oxidation of (A) gives an acid (B), C8H6O4. (B) easily forms anhydride on heating. Identify the compound (A).
1. 
2. 
3. 
4. 
Chlorobenzene is prepared commercially by:
1. Grignard reaction
2. Raschig process
3. Wurtz-Fittig reaction
4. Friedel-Crafts reaction










