The substances whose solubility decreases with increase in temperature:
1.
2.
3.
4. all of these
Two solutions containing and semipermeable membrane as shown below. If on reaction with produces blue colour of the blue colour will be noticed in:
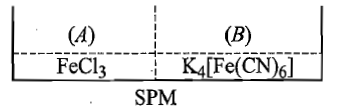
(1)
(2)
(3) in both and
(4) neither in nor in
Each pair forms ideal solution expect:
(1)
(2)
(3)
(4)
Mole fraction of solute in an aqueous solution that boils at 100.104 is :
( for = 0.52 K )
1. 0.008
2. 0.004
3. 0.061
4. 0.996
At Abu mountains, water boils at 96. What amount of NaCl be added in 1 kg water so that it boils at 100. .
1. 225 g
2. 450 g
3. 200 g
4. 125 g
Freezing point of an aqueous solution is -0.166C. Elevation of boiling point of same solution would be-
(Kb = 0.512 K m-1 and Kf = 1.66 K m-1)
| 1. | 0.18°C | 2. | 0.05°C |
| 3. | 0.09°C | 4. | 0.23°C |
A substance is completely trimerized on dissolution in a solvent. The van;t Hoff factor (i) for such change is:
1. 1
2. 2
3. 3
4. 1/3
The freezing point of aqueous solution that contains 5% by mass urea, 1.0% by mass KCl and 10% by mass of gulcose is: (kH=1.86 K molality)
1. 290.2 K
2. 285.5 K
3. 269.93 K
4. 250 K
The boiling point of an azeotropic mixture of water and ethyl alcohol is less than that of the theoretical value of a water and alcohol mixture. Hence, the mixture shows:
1. That solution is highly saturated
2. Positive deviation from Raoult's law
3. Negative deviation from Raoult's law
4. Nothing can be inferred
A maxima or minima obtained in the temperature, composition curve of a mixture of two liquids indicates:
1. an azeotropic mixture
2. a eutectic formation
3. that the liquids are immiscible with one another
4. that the liquids are partially miscible at hte maximum or minimum






