The adjoining graph shows change in conc. of substrate on enzyme activity. Identify A, B and C:

A
B
C
1.
Ki
Km
Vmax
2.
Km
Ki
3.
Vmax
Km
4.
Km
Vmax

A
B
C
1.
Ki
Km
Vmax
2.
Km
Ki
3.
Vmax
Km
4.
Km
Vmax
Go through the figures and select the correct option:
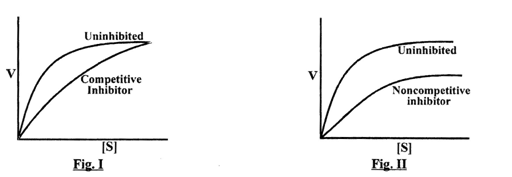
(1) I - In non-competitive type of enzymatic inhibition the V max decreases and Km remain unchanged
II - In competitive type of enzymatic inhibition the Vmax decreases and Km remain unchanged
(2) II - In non-competitive type of enzymatic inhibition the Vmax decreases and Km remain unchanged
I - In competitive type of enzymatic inhibition the Vmax remain same and Km is changed
(3) I - In non-competitive type of enzymatic inhibition the Vmax increases and Km remain unchanged
II - In competitive type of enzymatic inhibition the Vmax changes and Km decreases
(4) I - In non-competitive type of enzymatic inhibition the Vmax increases and Km remain unchanged
II - In competitive type of enzymatic inhibition the Vmax increases and Km increases
Assertion : The amino acid glycine comes under the category of nonessential amino acids.
Reason : This is due to the fact that it can not be synthesised in the body.
- If both the assertion and the reason are true and the reason is a correct explanation of the assertion
- If both the assertion and reason are true but the reason is not a correct explanation of the assertion
- If the assertion is true but the reason is false
- If both the assertion and reason are false
The figure given below shows the conversion of a substrate into product by an enzyme. In which one of the four options (a-d) the components of reaction labelled as A, B, C and D are identified correctly?
|
A |
B |
C |
D |
|
|
(1) |
Potential energy |
Transition state |
Activation energy with enzyme |
Activation energy without enzyme |
|
(2) |
Transition state |
Potential energy |
Activation energy without enzyme |
Activation energy with enzyme |
|
(3) |
Potential energy |
Transition state |
Activation energy with enzyme |
Activation energy without enzyme |
|
(4) |
Activation energy with enzyme |
Transition state |
Activation energy without enzyme |
Potential energy |
Amylase is an example of:
| 1. | Oxidoreductase | 2. | Transferase |
| 3. | Hydrolase | 4. | Ligase |
In competitive inhibition:
1. Inhibitor resembles the substrate in molecular structure
2. Competition between substrates and inhibitors to occupy active sites
3. Binding the inhibitors to active sites declines the enzyme action
4. All are correct
Choose the correct statement(s):
| 1. | Km (Michaelis - Menten) constant is the substrate concentration at which the enzymatic reaction attains half of its maximum velocity (1/2 Vmax) |
| 2. | At lower Km, higher the substrate affinity for enzyme |
| 3. | Vmax is reached when all the active sites of an enzyme are saturated with substrate |
| 4. | All are correct |
Which one of the following graphs show the relationship between the rate of an enzymatic activity and substrate conc.(S):
Which one of the graphs show the effect of temperature on the velocity of a typical enzymatic reaction?
Which one of the graphs shows the effect of pH on the velocity of a typical enzymatic reaction (V)?
1.












