A soap bubble is blown with the help of a mechanical pump at the mouth of a tube. The pump produces a certain increase per minute in the volume of the bubble, irrespective of its internal pressure. The graph between the pressure inside the soap bubble and time t will be:
1.

2.

3.

4.





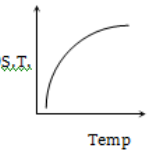
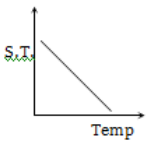
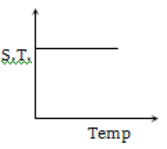
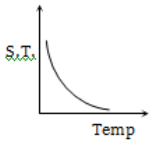
Which graph represents the variation of surface tension with temperature over small temperature ranges for water?
1. 
2. 
3. 
4. 
Two equal drops of water each of radius r are falling through air with a steady velocity
of 8 cm/s . The two drops combine to form a big drop. The terminal velocity of big
drop will be
(1)
(2)
(3)
(4) 32 cm/s
A ball of mass m and radius r is released in a viscous liquid. The value of its terminal
velocity varies linearly with
(1) 1/r only
(2) m/r
(3)
(4) m only
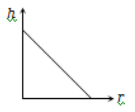
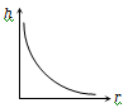
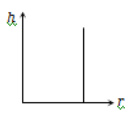
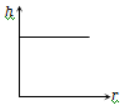
The correct curve between the height of depression h of liquid in a capillary tube and its radius is
1. 
2. 
3. 
4. 
In a surface tension experiment with a capillary tube, water rises upto 0.1 m. If the same experiment is repeated on an artificial satellite, which is revolving around the earth, water will rise in the capillary tube upto a height of-
(1) 0.1 m
(2) 0.2 m
(3) 0.98 m
(4) Full length of the capillary tube
A large number of water drops each of radius r combines to have a drop of radius R. If the surface tension is T and the mechanical equivalent of heat is J, then the rise in temperature will be
(1)
(2)
(3)
(4)
There is a horizontal film of soap solution. On it, a thread is placed in the form of a loop. The film is pierced inside the loop and the thread becomes a circular loop of radius \(R.\) If the surface tension of the loop is \(T,\) then what will be the tension in the thread?
1. \(\dfrac{πR^{2}}{T}\)
2. \(πR^{2} T\)
3. \(2 πRT\)
4. \(2 RT\)
Water rises against gravity in a capillary tube when its one end is dipped into water because
(1) Pressure below the meniscus is less than atmospheric pressure
(2) Pressure below the meniscus is more than atmospheric pressure
(3) Capillary attracts water
(4) Of viscosity
Kerosene oil rises up the wick in a lantern
(1) Due to surface tension of the oil
(2) The wick attracts the kerosene oil
(3) Of the diffusion of the oil through the wick
(4) None of the above






