Which compound in each of the following pairs will react faster in an \(S_N 2\) reaction with the −OH group?
(i) \(\mathrm{CH}_3 \mathrm{Br}\) or \(\mathrm{CH}_3 \mathrm{I}\)
(ii) \(\left(\mathrm{CH}_3\right)_3 \mathrm{CCl}\) or \(\mathrm{CH}_3 \mathrm{Cl}\)
Mark the correct option:
1. (i) \(\mathrm{CH}_3 \mathrm{Br}\) and (ii) \(\left(\mathrm{CH}_3\right)_3 \mathrm{CCl}\)
2. (i) \(\mathrm{CH}_3 \mathrm{I}\) and (ii) \(\mathrm{CH}_3 \mathrm{Cl}\)
3. (i) \(\mathrm{CH}_3 \mathrm{Br}\) and (ii) \(\mathrm{CH}_3 \mathrm{Cl}\)
4. (i)\(\mathrm{CH}_3 \mathrm{I}\)and (ii) \(\left(\mathrm{CH}_3\right)_3 \mathrm{CCl}\)
(ii) \(\left(\mathrm{CH}_3\right)_3 \mathrm{CCl}\) or \(\mathrm{CH}_3 \mathrm{Cl}\)
Mark the correct option:
1. (i) \(\mathrm{CH}_3 \mathrm{Br}\) and (ii) \(\left(\mathrm{CH}_3\right)_3 \mathrm{CCl}\)
2. (i) \(\mathrm{CH}_3 \mathrm{I}\) and (ii) \(\mathrm{CH}_3 \mathrm{Cl}\)
3. (i) \(\mathrm{CH}_3 \mathrm{Br}\) and (ii) \(\mathrm{CH}_3 \mathrm{Cl}\)
4. (i)\(\mathrm{CH}_3 \mathrm{I}\)and (ii) \(\left(\mathrm{CH}_3\right)_3 \mathrm{CCl}\)
In SN2 substitution reaction of the type
Which one of the following has the highest relative rate ?
(1)
(2)
(3)
(4)
Which one is the most reactive toward SN1 reaction?
1. C6H5CH(C6H5)Br
2. C6H5CH(CH3)Br
3. C6H5C(CH3)(C6H5)Br
4. C6H5CH2Br
The reaction of C6H5CH=CHCH3 with HBr produces
The compound that will react most readily with gaseous bromine has the formula ?
(1) C3H6
(2) C2H2
(3) C4H10
(4) C2H4
Consider the reaction
CH3CH2CH2Br + NaCN CH3CH2CH2CN + NaBr
This reaction will be the fastest in
1. ethanol
2. methanol
3. N,N'-dimethylformamide (DMF)
4. water
In the given reaction

the product P is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Which of the following can be used as the halide component for Friedel-Crafts reaction?
(1) Chlorobenzene
(2) Bromobenzene
(3) Chloroethene
(4) Isopropyl chloride
AgNO3(aq) gives yellow ppt. with:
(1) KIO3 (aq)
(2) KI (aq)
(3) CHI3
(4) CH2I2
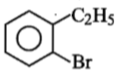
Compound (A), C8H9Br, gives a white precipitate when warmed with alcoholic AgNO3. Oxidation of (A) gives an acid (B), C8H6O4. (B) easily forms anhydride on heating. Identify the compound (A).
1. 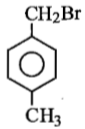
2. 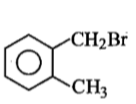
3. 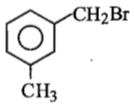
4.