A photon of Joules is absorbed by a material under special circumstances. The correct statement is:
1. Electrons of the atom of absorbed material will go the higher energy states
2. Electron and positron pair will be created
3. Only positron will be produced
4. Photoelectric effect will occur and electron will be produced
The maximum velocity of an electron emitted by light of wavelength incident on the surface of a metal of work function is
(1)
(2)
(3)
(4)
Where h = Planck's constant, m = mass of electron and c = speed of light.
In a photoemissive cell with executing wavelength , the fastest electron has speed v. If the exciting wavelength is changed to 3/4, the speed of the fastest emitted electron will be
(a) (b)
(c) Less than (d) Greater than
Photoelectric emission is observed from a metallic surface for frequencies and of the incident light rays . If the maximum values of kinetic energy of the photoelectrons emitted in the two cases are in the ratio of 1:k, then the threshold frequency of the metallic surface is
(1)
(2)
(3)
(4)
The ratio of de-Broglie wavelengths of molecules of hydrogen and helium which are at temperature 27 and 127 respectively is
(1)
(2)
(3)
(4) 1
A photon of wavelength 6630 Å is incident on a totally reflecting surface. The momentum delivered by the photon is equal to
(1) kg-m/sec
(2) kg-m/sec
(3) kg-m/sec
(4) None of these
The ratio of de-Broglie wavelength of a -particle to that of a proton being subjected to the same magnetic field so that the radii of their path are equal to each other assuming the field induction vector is perpendicular to the velocity vectors of the -particle and the proton is
1. 1
2.
3.
4. 2
In a photocell bichromatic light of wavelength 2475 Å and 6000 Å are incident on cathode whose work function is 4.8 eV. If a uniform magnetic field of Tesla exists parallel to the plate, the radius of the path described by the photoelectron will be (mass of electron = kg)
(1) 1 cm
(2) 5 cm
(3) 10 cm
(4) 25 cm
The figure shows the variation of photocurrent with anode potential for a photo-sensitive surface for three different radiations. Let and be the intensities and and be the frequencies for the curves a, b and c respectively. Then-
1.
2.
3.
4.
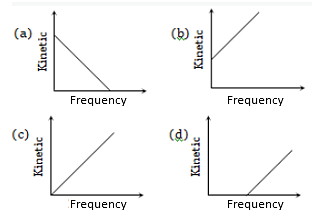
According to Einstein's photoelectric equation, the graph between the kinetic energy of photoelectrons ejected and the frequency of incident radiation is