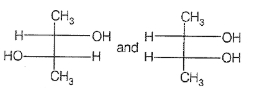
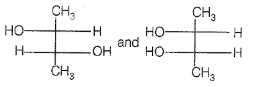
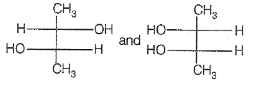
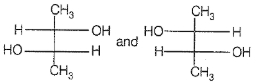Which of the following compounds is not chiral ?
1. DCH2CH2CH2Cl
2. CH3CH2CHDCl
3. CH3CHDCH2Cl
4. CH3CHClCH2D
A pair that represents chain isomer is:
1. CH3CHCl2 and ClCH2CH2Cl
2. Propyl alcohol and Isopropyl alcohol
3. 2-Methylbutane and Neopentane
4. Diethyl ether and Dipropylether
Number of monochlorinated products (excluding stereo-isomers) obtained from the given reaction are :

| 1. | 4 | 2. | 5 |
| 3. | 6 | 4. | 7 |
How many structural isomers are possible for the compound having molecular formula C3H5Br3?
1. 5
2. 4
3. 6
4. 8
Which of the following is an optically active compound?
1. 1-Butanol
2. 1-Propanol
3. 2-Chlorobutane
4. 4-Hydroxybutanal
In the following, the most stable conformation of n-butane is
1. 
2. 
3. 
4. 
The number of stereo isomers for 3-pentene 2-ol
1. 2
2. 4
3. 3
4. 5
The most stable configuration of n-butane will be
1. skew boat
2. eclipsed
3. gauche
4. staggered-anti
The compound having molecular formula C4H10O can show-
1. Metamerism
2. Functional isomerism
3. Positional isomerism
4. All of the above