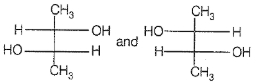How many structural isomers are possible for the compound having molecular formula C3H5Br3?
1. 5
2. 4
3. 6
4. 8
In the following, the most stable conformation of n-butane is
1. 
2. 
3. 
4. 
Which of the following acid does not exhibit optical isomerism?
1. Maleic acid
2. -amino acid
3. Lactic acid
4. Tartaric acid
The prefix used for -SH group as per the IUPAC system is-
1. Mercapto
2. Thiol
3. Sulfide
4. None of the above
Which of the following molecules exhibits chirality?
1. 2-Methylhexane
2. 3-Methylhexane
3. Neopentane
4. Isopentane
The property by virtue of which a compound can turn the plane of polarization of light is known as:
1. Photolysis
2. Phosphorescence
3. Optical activity
4. Polarization
Number of monochlorinated products (excluding stereo-isomers) obtained from the given reaction are :

| 1. | 4 | 2. | 5 |
| 3. | 6 | 4. | 7 |
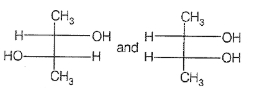
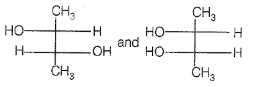
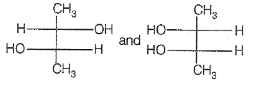
If optical rotation produced by the compound (i) is +52° then that produced by that the compound (ii) is:


1. -52°
2. +52°
3. 0°
4. unpredictable
But-2-ene exhibits cis-trans-isomerism due to
1. rotation around C2-C3 double bond
2. rotation around C3-C4 sigma bond
3. rotation around C1-C2 bond
4. restricted rotation around C=C bond