Which one of the following pairs represents stereoisomerism?
1. Chain isomerism and rotational isomerism
2. Structural isomerism and geometrical isomerism
3. Linkage isomerism and geometrical isomerism
4. Optical isomerism and geometrical isomerism
-D-glucose and -D glucose have a specific rotation of +112 and +19 respectively. In aqueous solution the rotation becomes +52. This process is called:
1. inversion
2. racemisation
3. mutarotation
4. embolism
A racemic mixture is formed by mixing two:
1. Isomeric compounds
2. Chiral compounds
3. Meso compounds
4. Enantiomers
A compound of molecular formula C7H16 shows optical isomerism, the compound will be
1. 2,3-dimethyl pentane
2. 2,2-dimethyl butane
3. 2-methyl hexane
4. None of the above
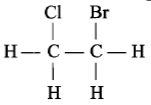
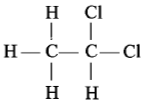
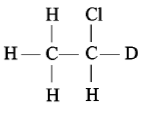
Which of the following has an asymmetric carbon atom?
1. 
2. 
3. 
4. 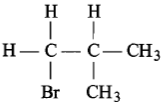
Stereoisomers (geometrical or optical) that are neither superimposable nor a mirror images to each other are called:
1. Enantiomers
2. Monomers
3. Tautomers
4. Diastereomers
Diastereomers can be separated by:
1. Recrystallization
2. simple distillation
3. electrophoresis
4. all of these
Which of the following pairs of carbon skeletons is an example of isomerism?
| 1. | 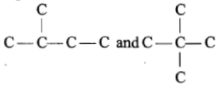  |
| 2. |
|
| 3. | 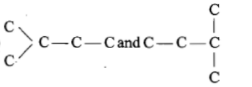  |
| 4. | 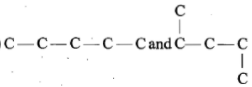  |
1. 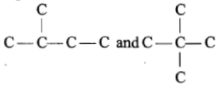
2. 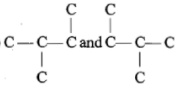
3. 
4. 
The number of isomeric structures for C2H7N would be:
1. 4
2. 3
3. 2
4. 1
Number of isomers possible for C4H8 are-
1. 4
2. 3
3. 2
4. 6







