Which one of the following is the stable structure of cyclohexatriene?
1. Chair form
2. Boat form
3. Half chair form
4. Planar form
Meso tartaric acid is optically inactive due to the presence of:
1. molecular symmetry
2. molecular asymmetry
3. external compensation
4. two asymmetric carbon atoms
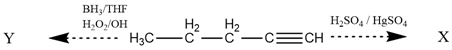 . Where X and Y are
. Where X and Y are
\(\xleftarrow{\mathrm {BH_3/THF\\{H_2O_2/OH}}}~\mathrm {H_3C-CH_2-CH_2-CH\xrightarrow{H_2SO_4/H_2SO_4}}~\mathrm X\\\)
1. Positional isomers
2. Functional isomers
3. Metamers
4. Tautomers
The property by virtue of which a compound can turn the plane of polarization of light is known as:
1. Photolysis
2. Phosphorescence
3. Optical activity
4. Polarization
The molecular formula of a saturated compound is C2H4Br2. This formula permits the existence of:
1. Functional isomers
2. Optical isomers
3. Positional isomers
4. Cis-trans isomers
Which of the following may exist as enantiomers?
| 1. |  |
| 2. | \(\mathrm{CH}_2=\mathrm{CHCH}_2 \mathrm{CH}_2 \mathrm{CH}_3 \) |
| 3. |  |
| 4. |  |
The total number of isomers could be obtained from the alkane, C6H14 is-
1. Four
2. Five
3. Six
4. Seven
\(\mathrm{CH_3-CHCl-CH_2-CH_3}\) has a chiral center. Which one of the following represents its R-configuration?
| 1. | 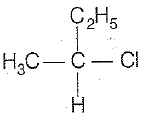  |
2. | 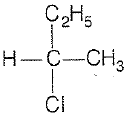  |
| 3. | 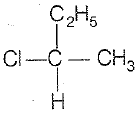  |
4. | 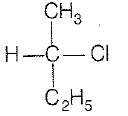  |
1. 
2. 
3. 
4. 
The number of primary carbon atoms in the following compound are:


| 1. | 6 | 2. | 2 |
| 3. | 4 | 4. | 3 |
4-methylpent-2-ene is achiral because it has:
1. a centre of symmetry
2. a plane of symmetry
3. symmetry at C 4 carbon
4. both centre and a plane of symmetry






