Which one of the following compounds is most acidic?
1. 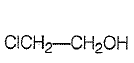
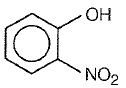
2. 
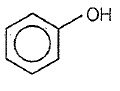
3. 
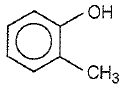
4. 
1.
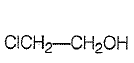

2.


3.


4.


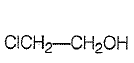



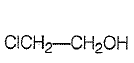






Which of the following represents the correct order of acidity in the given compounds?
1. FCOOH > COOH > BrCOOH > ClCOOH
2. BrCOOH > ClCOOH > FCOOH > COOH
3. FCOOH > ClCOOH > BrCOOH > COOH
4. COOH > BrCOOH > ClCOOH > FCOOH
Among the following, the strongest acid is:
| 1. | CH3COOH | 2. | CH2ClCH2COOH |
| 3. | CH2ClCOOH | 4. | CH3CH2COOH |
The distance between two adjacent carbon atoms is largest in
1. Benzene
2. Ethene
3. Butane
4. Ethyne
What is the correct order of increasing acidic strength among (I) p-methoxyphenol, (II) p-methylphenol, and (III) p-nitrophenol?
1. III < I < II
2. II < I < III
3. III < II < I
4. I < II < III
Which of the following represent the correct decreasing order of acidic strength of following?
(i) Methanoic acid
(ii) Ethanoic acid
(iii) Propanoic acid
(iv) Butanoic acid
1. (i)>(ii)>(iii)>(iv)
2. (ii)>(iii)>(iv)>(i)
3. (i)>(iv)>(iii)>(ii)
4. (iv)>(i)>(iii)>(ii)
The IUPAC name of the compound,
 is:
is:
1. 1, 3-Dibromo-3-methylbutane
2. 3-Methyl-1,2-bromobutane
3. 3-Methyl-1,3-bromopropane
4. None of the above
Which one of the following has the shortest carbon-carbon bond length?
1. Benzene
2. Ethene
3. Ethyne
4. Ethane
Which of the following is more basic than aniline?
1. Diphenylamine
2. Triphenylamine
3. p-nitroaniline
4. Benzylamine
The shortest C-C bond distance is found in
1. Diamond
2. Ethane
3. Benzene
4. Acetylene






